儿茶酚催化的烷基硼酸与醛和胺的光催化羰基化四组分反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
羰基化反应利用CO作为易于获得的Cl源,已经成为从现成的起始材料构建含羰基化合物的最有力的工具之一。尽管在羰基化转化方面取得了令人印象深刻的成就,但这些方法的一个基本限制在于它们依赖于过渡金属催化或活性试剂的化学计量量。本文报道了第一个光诱导儿茶酚催化烷基硼酸与醛和胺的四组分羰基化反应。以中高收率制备了一系列有价值的取代α-氨基酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
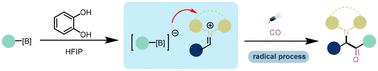
A catechol-catalyzed photocatalytic carbonylative four-component reaction of alkylboronic acids with aldehydes and amines†
Carbonylation reactions employing CO as an easily accessible Cl source have emerged as one of the most powerful tools for constructing carbonyl-containing compounds from readily available starting materials. Despite the impressive achievements on carbonylative transformations, a fundamental limitation of these approaches lies in their reliance on transition metal catalysis or a stoichiometric amount of activating reagents. Herein, we report the first photo-induced catechol-catalyzed four-component carbonylation reaction of alkylboronic acids with aldehydes and amines. A range of valuable substituted α-amino ketones was produced in moderate to good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


