吩噻嗪类有机光氧化还原催化研究进展
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
近年来,光氧化还原催化因其在可见光诱导的有机转化中的广泛适用性而引起了广泛的关注。虽然高氧化性催化剂(如吖啶催化剂)的开发取得了重大进展,但强还原性有机光氧化还原催化剂仍显着不足。吩噻嗪类由于其独特的氧化还原电位,特别是其低激发态氧化电位(Eox* = - 1.35 V ~ - 3.51 V vs. SCE)而被广泛用作光氧化还原催化剂。因此,它们可以应用于多种具有氧化猝灭循环的光氧化还原反应,并有效还原各种有机分子,如芳基和烷基卤化物、烯烃、丙二酰过氧化物、钴配合物和氧化还原活性酯。鉴于其独特的性质,本文综述了近年来吩噻嗪类有机光氧化还原催化的研究进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
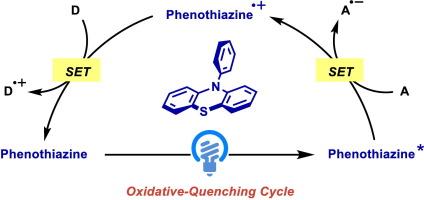
Recent progress on phenothiazine organophotoredox catalysis
Photoredox catalysis has garnered significant attention in recent years due to its broad applicability in visible-light-induced organic transformations. While significant progress has been made in the development of highly oxidizing catalysts, such as acridinium catalysts, there remains a notable shortage of strongly reducing organophotoredox catalysts. Phenothiazines are widely used as photoredox catalysts owing to their unique redox potentials, particularly their low excited-state oxidation potentials (Eox* = −1.35 V to −3.51 V vs. SCE). Thus, they can be applied to a variety of photoredox reactions with oxidative-quenching cycles, and effectively reduce various organic molecules, such as aryl and alkyl halides, alkenes, malonyl peroxides, cobalt complexes, and redox-active esters. Due to their unique properties, this review focuses on the recent advances in phenothiazine organophotoredox catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


