烯烃三组分碳卤化反应中作为碳自由基前体的亚砜酰亚砜
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们开发了一种新的可见光诱导光氧化还原平台,用于以亚砜酰亚砜为碳自由基前体的烯烃的一般三组分双官能化。该反应利用各种烯烃作为底物,并采用具有成本效益、无毒的金属盐作为卤素供体。该工艺在温和的水条件下进行,不需要酸或碱添加剂,为合成具有优异化学选择性和区域选择性的结构多样的富含C(sp3)的有机卤化物提供了一种简单而环保的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
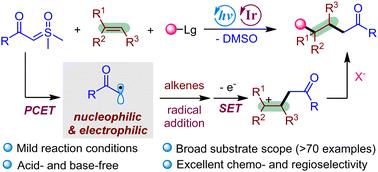
Sulfoxonium ylides as carbon radical precursors in three-component carbohalogenation of alkenes†
Herein, we developed a novel visible-light-induced photoredox platform for general three-component difunctionalization of alkenes with aqueous sulfoxonium ylides as carbon radical precursors. This reaction utilizes various alkenes as substrates and employs cost-effective, non-toxic metal salts as halogen donors. The process proceeds under mild aqueous conditions without requiring acid or base additives, offering a simple and environmentally benign strategy for the synthesis of structurally diverse C(sp3)-rich organic halides with excellent chemo- and regioselectivity profiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


