一种抑制肝癌生长的新型HSP90AB1抑制剂的鉴定
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肝细胞癌(HCC)仍然是全球癌症相关死亡的主要原因,治疗选择有限。从鸦嘴鸦属植物中提取的天然化合物,如鸦嘴鸦素A (BRA),已成为很有前景的抗癌药物。然而,其在HCC中的作用机制在很大程度上仍未被探索。目的确定BRA的分子靶点,阐明其抗癌作用。方法采用患者源性类器官(PDOs)和异种移植器官(PDX)模型评价BRA的抗癌活性。通过MST、SPR和CETSA等化学蛋白质组学和结合分析,确定热休克蛋白90α家族B类成员1 (HSP90AB1)为BRA的主要靶点,SER-108为关键结合位点。HSP90AB1敲低进一步证实了其在bra介导的抗hcc作用中的关键作用。此外,基于tmt的蛋白质组学研究了HSP90AB1的下游伴侣蛋白。结果bra能明显抑制肝癌细胞增殖,诱导细胞凋亡。分子分析显示HSP90AB1为关键靶点,SER-108为关键结合位点。蛋白质组学分析发现下游HSP90AB1伴侣蛋白,包括PIK3CG、EGFR和KDM5C,是BRA抑制HCC进展的因素。结论本研究确定HSP90AB1为BRA的主要靶点,其通过调控下游伴侣蛋白PIK3CG、EGFR、KDM5C发挥抗hcc作用。这些发现突出了BRA在HCC中的治疗潜力,并表明HSP90AB1代表了未来药物开发的一个有希望的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
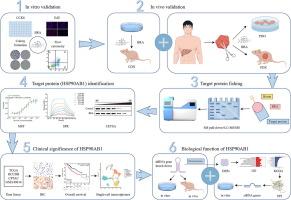
Identification of Bruceine A as a novel HSP90AB1 inhibitor for suppressing hepatocellular carcinoma growth
Introduction
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide, with limited therapeutic options available. Natural compounds, such as Bruceine A (BRA), derived from Brucea javanica, have emerged as promising anticancer agents. However, the underlying mechanisms of action in HCC remain largely unexplored.Objectives
This study aims to identify the molecular target of BRA and elucidate its anticancer effects.Methods
Patient-derived organoids (PDOs) and xenograft (PDX) models were employed to assess the anticancer activity of BRA. Chemical proteomics and binding assays, including MST, SPR, and CETSA, facilitated the identification of heat shock protein 90α family class B member 1 (HSP90AB1) as the primary target of BRA, with SER-108 identified as the critical binding site. HSP90AB1 knockdown further confirmed its pivotal role in BRA-mediated anti-HCC effects. Additionally, TMT-based proteomics was applied to investigate the downstream chaperones of HSP90AB1.Results
BRA significantly suppressed HCC proliferation and induced apoptosis. Molecular analyses revealed HSP90AB1 as the key target, with SER-108 as the critical binding site. Proteomic analysis identified downstream HSP90AB1 partner proteins, including PIK3CG, EGFR, and KDM5C, as contributors to BRA’s inhibitory effects on HCC progression.Conclusion
This study establishes HSP90AB1 as the primary target of BRA, which exerts its anti-HCC effects through modulation of downstream chaperones PIK3CG, EGFR, and KDM5C. These findings highlight the therapeutic potential of BRA in HCC and suggest that HSP90AB1 represents a promising target for future drug development.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


