IL-4损害皮肤驻留记忆CD8+ T细胞的形成
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
局部细胞因子,包括TGFβ,驱动CD8+组织驻留记忆T (TRM)细胞在组织内的分化和长期持久性。然而,阻止CD8+ TRM细胞形成的信号尚未明确。在这里,我们发现IL-4抑制CD8+ T细胞获得上皮TRM细胞表型。IL-4通过激活CD8+ T细胞,抑制tgf β诱导的CD103和CD49a的表达,提高Eomes的表达。这种表型变化与tgf - β rii的持续下调、pSmad2/3的表达减少和抑制性Smad7的表达增加有关。在激活过程中暴露于IL-4的初始CD8+ T细胞表现出皮肤CD103+CD8+ TRM细胞形成受损。此外,特应性皮炎病变内产生的IL-4降低了浸润性CD8+ T细胞中CD103的表达,减少了CD8+ TRM细胞的形成,从而降低了对皮肤单纯疱疹病毒感染的保护作用。总之,这些发现表明,IL-4降低了CD8+ T细胞对TGFβ的反应性,导致CD8+ TRM细胞的形成受损,CD8+ TRM细胞介导的局部感染保护受损。本文章由计算机程序翻译,如有差异,请以英文原文为准。
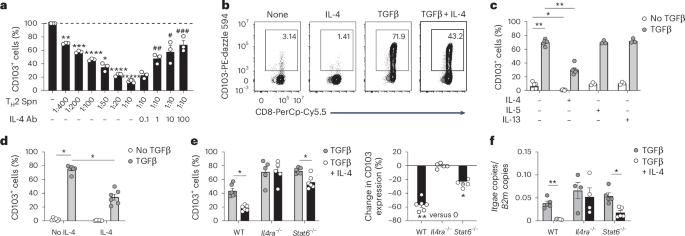

IL-4 impairs the formation of skin-resident memory CD8+ T cells
Local cytokines, including TGFβ, drive CD8+ tissue-resident memory T (TRM) cell differentiation and long-term persistence within tissues. However, the signals that prevent CD8+ TRM cell formation are not well defined. Here we found that IL-4 suppressed CD8+ T cell acquisition of an epithelial TRM cell phenotype. IL-4 inhibited the expression of TGFβ-induced CD103 and CD49a and increased the expression of Eomes by activated CD8+ T cells in vitro and in vivo. This change in phenotype was correlated with prolonged downregulation of TGFβRII, decreased expression of pSmad2/3 and increased expression of inhibitory Smad7. Naive CD8+ T cells exposed to IL-4 during activation exhibited impaired cutaneous CD103+CD8+ TRM cell formation. Additionally, IL-4 produced within atopic dermatitis lesions decreased the expression of CD103 in infiltrating CD8+ T cells and reduced CD8+ TRM cell formation, resulting in reduced protection from cutaneous herpes simplex virus infection. Together, these findings reveal that IL-4 decreases the responsiveness of CD8+ T cells to TGFβ, resulting in impaired formation of CD8+ TRM cells and impaired CD8+ TRM cell-mediated protection from local infection. Bromley and colleagues show that IL-4 decreases the responsiveness of CD8+ T cells to TGFβ, resulting in impaired formation of CD8+ TRM cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


