ZnO纳米棒阵列在乙醇检测中的气体响应机理研究
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
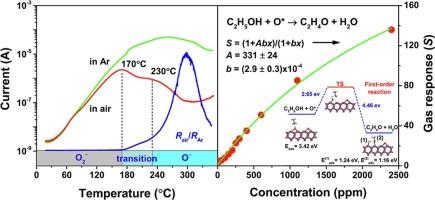
氧化锌传感器的气体响应机制是一个尚未得到很好解决的问题。本文采用第一性原理计算方法,研究了氧在ZnO(10¯0)表面的吸附以及乙醇与化学吸附的O (O*)原子的反应。研究发现,在170 °C ~ 350 °C温度下,O*是主导ZnO纳米棒电导率变化的主要物质,在 ~ 300 °C温度下覆盖范围最大,这与气体传感器的最佳工作温度一致。化学吸附O2和O的未占据的定域轨道位于带隙区,从而导致电子在导带和氧之间转移。吸附在O*位的乙醇会被氧化成乙醛和水,由于吸附能小,乙醛和水是主要的气相产物。在一阶反应条件下,得到了气体响应方程,并拟合出了检测不同浓度乙醇的气体响应曲线。ZnO纳米棒在O*位点上对乙醇、H2、CO和CH4的吸附差异是ZnO纳米棒气体选择性的主要原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Study on the gas response mechanism of ZnO nanorod arrays in the detection of ethanol
The gas response mechanism of ZnO sensors is an issue that is not well addressed. In this work, the adsorption of oxygen species on the (10 0) surface of ZnO and the reaction of ethanol with chemisorbed O (O*) atoms are studied by a method of first-principles calculations. It is found that O* is the major species that dominates the variation in the conductivity of ZnO nanorods at the temperatures of 170 °C to 350 °C and the largest coverage appears at ∼300 °C, which is in agreement with the best working temperature of gas sensors. The unoccupied localized orbitals of chemisorbed O2 and O are in the band-gap region and thus lead to the electron transfer between the conduction band and oxygen species. Ethanol adsorbed at O* sites will be oxidized into acetaldehyde and water, which are the major vapor-phase products due to the small adsorption energy. Under the first-order reaction, a formula is obtained and the curve of gas response in detecting ethanol with different concentrations is well fitted. The difference in the adsorption of ethanol, H2, CO, and CH4 at O* sites is found a reason dominating the gas selectivity of ZnO nanorods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


