急性冷胁迫下黄鳍金枪鱼幼鱼脾脏组织损伤及生理反应机制
IF 2.4
3区 农林科学
Q1 FISHERIES
引用次数: 0
摘要
全球气候变化导致的海水温度异常严重破坏了海洋鱼类的生理稳态和免疫调节。黄鳍金枪鱼(Thunnus albacares)是一种温血的远洋鱼类,虽然具有一定的体温调节能力,但在突冷环境下仍会经历显著的生理应激。脾脏是人体重要的免疫和代谢器官,对温度波动高度敏感,是冷应激效应的重要指标。本研究将黄鳍金枪鱼幼鱼分别置于24°C (LT组)和18°C (ULT组)低温胁迫下,30°C作为对照组(CG组)。分别在0、12、24和36 h取样。通过评估脾脏抗氧化酶和代谢酶活性、组织病理变化和免疫基因表达谱,我们系统地评估了不同冷强度下的组织损伤和生理反应。结果显示,急性冷应激引起了显著的脾损伤,包括核变形、空泡化、黑素-巨噬细胞聚集,其中以ULT组损伤最为严重。抗氧化反应显示,两个冷应激组在36 h时CAT活性显著升高,在0 h和36 h时MDA水平显著升高(p <;0.05)。ALT、AST、LDH、ACP等代谢酶表现出动态波动,在36 h时,ULT组ACP活性显著升高。免疫相关基因(hspa8b、irf3、b2m、blmh)表现出时间和温度依赖性表达,在24 h时表达上调,在36 h时部分下调,表明免疫激活后出现潜在抑制。这些发现强调了黄鳍金枪鱼脾脏免疫代谢轴对冷应激的脆弱性,并为理解区域恒温海洋鱼类温度诱导的生理功能障碍提供了重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
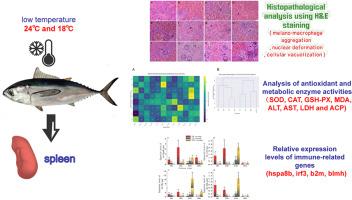
Splenic tissue injury and physiological response mechanisms in juvenile yellowfin tuna (Thunnus albacares) under acute cold stress
Abnormal seawater temperatures driven by global climate change are profoundly disrupting the physiological homeostasis and immune regulation of marine fish. As a warm-blooded pelagic species, yellowfin tuna (Thunnus albacares) possesses partial thermoregulatory capability but still experiences significant physiological stress under abrupt cold exposure. The spleen, a key immune and metabolic organ, is highly sensitive to temperature fluctuations and serves as a critical indicator of cold stress effects. In this study, juvenile yellowfin tuna were subjected to cold stress at 24 °C (LT group) and 18 °C (ULT group), with 30 °C as the control (CG group). Sampling was conducted at 0, 12, 24, and 36 h. By evaluating splenic antioxidant and metabolic enzyme activities, histopathological changes, and immune gene expression profiles, we systematically assessed tissue injury and physiological responses under different cold intensities. Results showed that acute cold stress induced notable splenic damage, including nuclear deformation, vacuolization, and melano-macrophage aggregation, with the most severe lesions observed in the ULT group. Antioxidant responses revealed significantly elevated CAT activity at 36 h and increased MDA levels at 0 h and 36 h in both cold-stressed groups (p < 0.05). Metabolic enzymes such as ALT, AST, LDH, and ACP exhibited dynamic fluctuations, with ACP activity significantly increased at 36 h in the ULT group. Immune-related genes (hspa8b, irf3, b2m, blmh) displayed time- and temperature-dependent expression, with upregulation at 24 h and partial downregulation at 36 h, indicating immune activation followed by potential suppression. These findings highlight the vulnerability of the splenic immune-metabolic axis in yellowfin tuna to cold stress and offer important implications for understanding temperature-induced physiological dysfunction in regionally endothermic marine fish.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.20
自引率
6.90%
发文量
206
审稿时长
49 days
期刊介绍:
Developmental and Comparative Immunology (DCI) is an international journal that publishes articles describing original research in all areas of immunology, including comparative aspects of immunity and the evolution and development of the immune system. Manuscripts describing studies of immune systems in both vertebrates and invertebrates are welcome. All levels of immunological investigations are appropriate: organismal, cellular, biochemical and molecular genetics, extending to such fields as aging of the immune system, interaction between the immune and neuroendocrine system and intestinal immunity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


