一种新型掺磷Ge3N4粉末作为锂离子电池高容量负极材料
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文研究了磷原子(P原子)掺杂对Ge3N4作为锂离子电池(LIBs)负极材料电化学性能的影响。以尿素和NaH2PO2·H2O为氮磷源,采用原位氮化磷化法制备了p掺杂Ge3N4 (P-Ge3N4)。系统地表征了P-Ge3N4材料,研究了磷掺杂对Ge3N4结构的影响。测试了P-Ge3N4负极材料在锂离子电池中的电化学性能。结果表明,P原子的存在提高了整体的电子导电性。P-Ge3N4材料的首次放电容量为1548.53 mAh g−1,远高于纯Ge3N4材料的850.26 mAh g−1,并且在100次循环后P-Ge3N4仍保持比Ge3N4更高的可逆放电容量。与Ge3N4相比,P-Ge3N4表现出优越的倍率性能,在不同电流密度下保持较高的放电容量。通过密度泛函理论(DFT)模拟和计算,发现带隙宽度从1.76 eV减小到0.27 eV,表明P掺杂提高了Ge3N4的电导率。本研究通过实验表征和理论计算相结合,建立了一种有效的磷原子掺杂策略,展示了P的引入如何改变Ge3N4的晶体结构、电子性能和电化学性能。为高性能锂离子电池负极材料的设计和优化提供了新的理论和实验依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
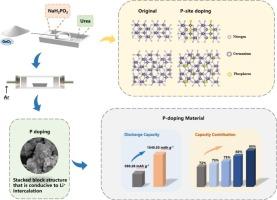
A novel phosphorus-doped Ge3N4 powder as high-capacity anode materials for lithium-ion batteries
In this paper, the effect of doping with phosphorus atoms (P atoms) on the electrochemical performance of Ge3N4 as anode materials for lithium-ion batteries (LIBs) is investigated. P-doped Ge3N4 (P-Ge3N4) is synthesized by an in-situ nitridation and phosphorization method utilizing urea and NaH2PO2·H2O as nitrogen and phosphorus sources. The P-Ge3N4 material is systematically characterized and the effect of phosphorus doping on the structure of Ge3N4 is studied. The electrochemical performance of the P-Ge3N4 anode materials in LIBs is also tested. The results indicate that the presence of P atoms enhances the overall electronic conductivity. The first discharge capacity of P-Ge3N4 material is 1548.53 mAh g−1, which is much higher than that of pure Ge3N4 (850.26 mAh g−1), and P-Ge3N4 still maintains a higher reversible discharge capacity than Ge3N4 after 100 cycles. P-Ge3N4 exhibits superior rate capability compared to Ge3N4, maintaining higher discharge capacities at various current densities. Based on density functional theory (DFT) simulation and calculation, it is found that the band gap width decreased from 1.76 eV to 0.27 eV, indicating that P doping improves the conductivity of Ge3N4. This study establishes an effective phosphorus atom doping strategy by combining experimental characterization and theoretical calculations, showing how the introduction of P changes the crystal structure, electronic properties and electrochemicl performance of Ge3N4. It provides a new theoretical and experimental basis for the design and optimization of high-performance anode materials for LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


