甲烷干重整强化制氢Ni催化剂的合成与评价:响应面法研究
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
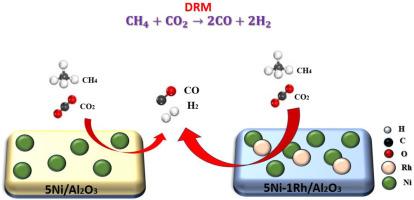
干重整甲烷(DRM)是一种生产氢气的方法,具有很强的推广意义和环保意义。除了通过氢燃料提供清洁能源生产的途径外,它还通过同时使用两种强大的温室气体:CO₂和CH₄提供了双重环境优势。在700°C下,采用一系列5% ni基催化剂,以1% Rh, La, Au和Sm为催化剂,负载在氧化铝上,对DRM进行了测试。采用浸渍法制备了催化剂。采用N₂物理吸附、H₂-TPR、XRD、CO₂-TPD、拉曼光谱、TEM和XPS等测试手段对其结构质量、还原性、结构特征、碱度、碳沉积、形貌和表面化学状态进行了表征。Rh的加入显著提高了催化活性,H₂和CO的产率最高(分别约为60%和70%),H₂/CO比值为0.85。然而,与未促进的5Ni/Al₂O₃催化剂相比,La、Au和Sm的加入导致催化性能下降,突出了促进剂效果的选择性。表征研究表明,La、Au和Sm促进了焦炭沉积,拉曼光谱证实了这一点,而Rh促进了更高的还原性,改善了Ni纳米颗粒的分散性。对废5Ni-Rh/Al2O3催化剂的TEM研究表明,碳纳米管和纳米纤维包裹了活性位点。XPS分析表明,新鲜的5Ni/Al2O3和5Ni- rh /Al2O3催化剂中均存在NiAl₂O4。铑促进的催化剂表现出向更高结合能的适度转变,表明金属-载体相互作用得到改善。利用响应面法,研究了温度、空速(SV)和硫酸铵与CO₂的比对CO和H₂产率及其比值的影响。研究结果表明,最重要的成分是温度,它显著增加了CO和H₂的输出。降低SV和CH₄/CO₂比也提高了产率。结果表明,H₂/CO比随温度升高和SV降低而增大,但随温度升高和CH₄/CO₂均增大。在DRM中,通过数值优化确定了1% rh促进Ni催化剂的理想条件,H2产率在95%以上。实验结果与理论预期非常吻合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and evaluation of promoted Ni catalysts for enhanced hydrogen production in dry reforming of methane: A response surface methodology study
Dry reforming methane (DRM) is a method that produces hydrogen, which is highly promotional and environmentally relevant. In addition to providing a route to cleaner energy production via hydrogen fuel, it offers a double environmental advantage by utilizing two powerful greenhouse gases at the same time: CO₂ and CH₄. The DRM at 700 °C was tested using a series of 5 % Ni-based catalysts promoted with 1 % Rh, La, Au, and Sm and supported on alumina. The catalysts were prepared via the impregnation method. The textural qualities, reducibility, structural features, basicity, carbon deposition, morphology, and surface chemical states were examined using N₂ physisorption, H₂-TPR, XRD, CO₂-TPD, Raman spectroscopy, TEM, and XPS. Rh addition significantly increased the catalytic activity, resulting in the highest H₂ and CO yields (approximately 60 % and 70 %, respectively) and an H₂/CO ratio of 0.85. However, when compared to the unpromoted 5Ni/Al₂O₃ catalyst, the addition of La, Au, and Sm resulted in a decline in catalytic performance, highlighting the selectivity of promoter effectiveness. According to characterization investigations, La, Au, and Sm promotion increased coke deposition, which was validated by Raman spectroscopy, while Rh promotion promoted higher reducibility and improved the dispersion of Ni nanoparticles. TEM investigation of the spent 5Ni-Rh/Al2O3 catalyst revealed that carbon nanotubes and nanofibers encapsulated the active sites. XPS analysis revealed the presence of NiAl₂O4 species in both fresh 5Ni/Al2O3 and 5Ni-Rh/Al2O3 catalysts. The Rh-promoted catalyst showed a modest shift towards higher binding energies, indicating improved metal-support interactions. Using response surface methods, this study examines how temperature, space velocity (SV), and the ratio of CH₄ to CO₂ affect the CO and H₂ yields and their ratios. The findings indicate that the most important component is temperature, which significantly increases the CO and H₂ output. These yields are also improved by lowering SV and the CH₄/CO₂ ratio. As a result, the H₂/CO ratio increased with higher temperature and lower SV, but it also increased with both temperature and CH₄/CO₂. In DRM, the ideal conditions for the 1 % Rh-promoted Ni catalyst were determined through numerical optimization, yielding over 95 % H2 yield. The experimental findings closely matched the theoretical expectations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


