碱性条件下用于析氢反应的镍铁基电催化剂:设计和构效关系的最新趋势
IF 23.8
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
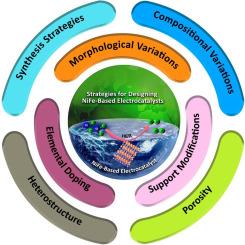
使用“绿色氢”作为零碳排放的未来燃料,是化石燃料的最佳替代品之一,也是解决碳排放和能源危机等与化石燃料永续消耗有关的问题的合理解决方案。电催化水分解反应产生“绿色氢”具有很高的动能垒,因此开发一种高性能的电催化剂是非常关键和具有挑战性的。基于NiFe催化剂体系的电催化剂由于其成本低、易于获得、比纯镍和纯铁材料具有更多的电化学活性表面位点以及镍和铁之间的协同作用而具有优异的电子性能而受到广泛关注。本文综述了近年来的发展趋势,并综合分析了文献中描述的设计和优化碱性介质中有效的nife基析氢反应(HER)电催化剂的关键因素。从实验和理论两方面论述了影响镍铁基电催化剂催化效率的重要因素,如催化剂表面形貌、电子结构、载体材料特性、金属或非金属杂原子掺杂、异质结构、合成策略、组成变化和孔结构等。这些参数的变化使催化剂的活性位点暴露较多,比表面积增大,电导率提高,质量扩散快,氢气易于从催化剂表面解吸,稳定性好。本文还讨论了在碱性条件下电催化水裂解反应中,基于nife的整体水裂解,以及用于阐明反应机理和催化剂结构演变的各种原位/operando研究。最后指出了在碱性介质下发展镍铁基HER电催化剂所面临的挑战和前景。尽管电催化HER领域已经取得了进展,但仍需要不断努力,制造出一种高效的基于nfe的电催化剂,这种电催化剂必须具有长期的电化学稳定性,并具有可持续性的氢气生产和商业应用的可扩展性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
NiFe-based electrocatalysts for hydrogen evolution reaction in alkaline conditions: Recent trends in the design and structure–activity correlations
One of the best alternatives to fossil fuels and a plausible solution to the issues related to its perpetual consumption such as carbon emission and energy crisis is the use of “green hydrogen” as the fuel of future with zero carbon emission. The electrocatalytic water-splitting reaction to produce ‘green hydrogen’ has a high kinetic energy barrier and hence developing a high performance electrocatalyst is very crucial and challenging. The electrocatalysts that based on NiFe catalyst system has received considerable attention because of their low cost, easy availability, increased electrochemically active surface sites compared to pure nickel and iron materials, and excellent electronic properties due to the synergistic interaction between Nickel and Iron. This review highlights the recent trends and a comprehensive analysis of the critical factors described in the literature for the design and optimization of an effective NiFe-based hydrogen evolution reaction (HER) electrocatalyst in alkaline medium. The important factors that influence the catalytic efficiency of NiFe-based electrocatalysts such as the modifications in the surface morphology, electronic structure of the catalyst, supporting material characteristics, doping with heteroatoms of metals or non-metals, heterostructuring, synthesis strategies, compositional variations, and pore structure of the catalyst are addressed from experimental and theoretical point of view. The variation of these parameters provides much exposed active sites, improved surface area, electronic conductivity, fast mass diffusion and easy desorption of hydrogen gas from the catalyst surface and stability. The NiFe-based overall water splitting, and various in situ/operando studies employed for elucidating the reaction mechanism as well as the structural evolution of the catalyst during the electrocatalytic water splitting reaction under alkaline conditions are also discussed in this review. The challenges and prospects for developing NiFe-based electrocatalyst for HER under alkaline medium are highlighted in the end. Even though advancement has made in the area of electrocatalytic HER, continuous efforts are needed to fabricate a highly efficient NiFe-based electrocatalyst that show long term electrochemical stability along with scalability for sustainable H2 production and implementation of it for commercial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

EnergyChem
Multiple-
CiteScore
40.80
自引率
2.80%
发文量
23
审稿时长
40 days
期刊介绍:
EnergyChem, a reputable journal, focuses on publishing high-quality research and review articles within the realm of chemistry, chemical engineering, and materials science with a specific emphasis on energy applications. The priority areas covered by the journal include:Solar energy,Energy harvesting devices,Fuel cells,Hydrogen energy,Bioenergy and biofuels,Batteries,Supercapacitors,Electrocatalysis and photocatalysis,Energy storage and energy conversion,Carbon capture and storage
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


