BRD4770通过抑制细胞凋亡和铁下垂来保护dox诱导的心脏毒性
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
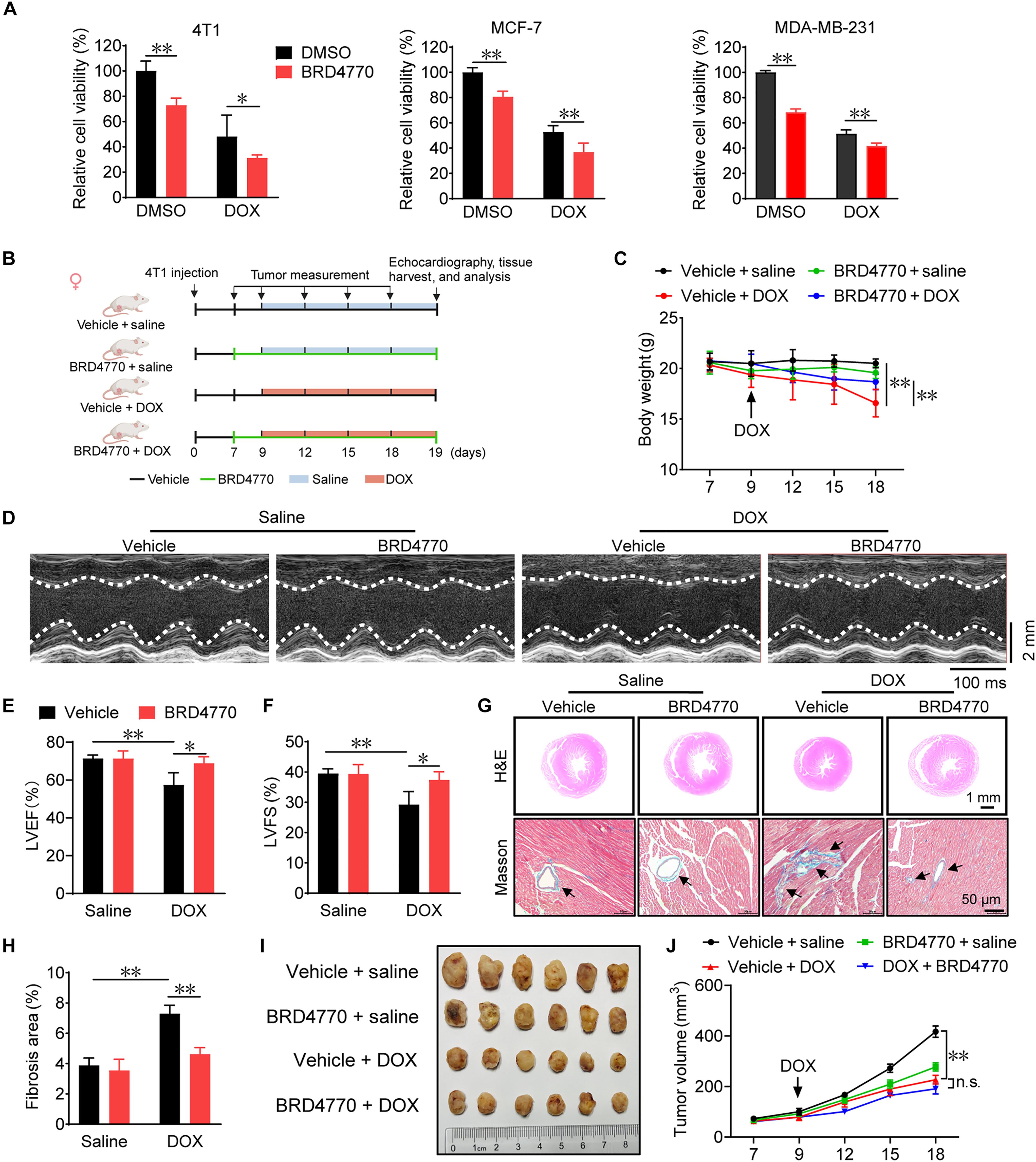
多柔比星(DOX)是一种有效的抗癌药物,但其临床应用主要受到心肌细胞凋亡和铁中毒的限制。虽然dox诱导的心脏毒性(DIC)涉及表观遗传改变,但尚未有系统的表观遗传干预研究报道。在这里,我们通过表观遗传学化合物文库筛选发现BRD4770是一个潜在的抗DIC的小分子。BRD4770通过减少活性氧(ROS)的产生和脂质过氧化以及维持谷胱甘肽的稳态来抑制dox诱导的心肌细胞凋亡。BRD4770治疗可减轻DIC,但不影响DOX的抗肿瘤作用。在机制上,BRD4770通过降低组蛋白3上赖氨酸9的甲基化,促进核因子红系2相关因子2 (Nrf2)/激活转录因子4 (ATF4) -溶质载体家族7成员11 (SLC7A11)信号传导,抑制dox诱导的心肌细胞死亡。最后,我们构建了一个ros响应的纳米脂质体,装载BRD4770并与原代心肌细胞的心脏靶向肽结合,对DIC有更好的保护作用。这些发现表明BRD4770具有预防心肌细胞死亡相关心肌病的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
BRD4770 protects against DOX-induced cardiotoxicity by inhibiting apoptosis and ferroptosis
Doxorubicin (DOX) is an effective anticancer drug, but its clinical utility is limited mainly by cardiotoxicity causing cardiomyocyte ferroptosis and apoptosis. While DOX-induced cardiotoxicity (DIC) involves epigenetic changes, no systematic epigenetic intervention studies have been reported. Here, we identified BRD4770 as a potential small molecule against DIC through the Epigenetics Compound Library screening. BRD4770 inhibited DOX-induced cardiomyocyte ferroptosis and apoptosis by reducing reactive oxygen species (ROS) production and lipid peroxidation and maintaining glutathione homeostasis. BRD4770 treatment alleviated DIC without affecting the antitumor effects of DOX. Mechanistically, BRD4770 promoted nuclear factor erythroid 2-related factor 2 (Nrf2)/activating transcription factor 4 (ATF4)–solute carrier family 7 member 11 (SLC7A11) signaling and suppressed DOX-induced cardiomyocyte death by reducing methylation of lysine 9 on histone 3. Last, we constructed a ROS-responsive nanoliposome loaded with BRD4770 and conjugated with the cardiac-targeting peptide for primary cardiomyocyte, which provided better protection against DIC. These findings suggest that BRD4770 has the potential to prevent cardiomyocyte death–related cardiomyopathy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


