单独佩戴噬菌体和可装载药物的黏附纳米头盔,用于治疗眼部感染
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
眼部感染造成明显的失明风险。尽管噬菌体疗法在抑制多药耐药细菌和消除生物膜方面具有优势,但它存在噬菌体活力低、眼潴留有限以及对眼部感染缺乏抗炎能力的问题。在这里,用可粘附的载药纳米头盔单独佩戴噬菌体可以促进噬菌体治疗。通过静电相互作用将精氨酸-甘氨酸-天冬氨酸修饰的壳聚糖沉积在带负电荷的噬菌体头上,然后通过物理吸附共沉积抗炎剂形成纳米头盔。纳米头盔的形成对噬菌体活力的影响可以忽略不计,适用于各种头盔噬菌体。由于其阳离子性质和精氨酸-甘氨酸-天冬氨酸序列的存在,这种纳米头盔在眼表面表现出双锁的粘附方式,在注入后延长了噬菌体的滞留时间。与持续的药物释放一起,头盔噬菌体有效地抑制细菌,消除生物膜,有效地抑制局部炎症。在患有多重耐药病原体诱导的角膜炎的小鼠中,头盔噬菌体取得了优于临床治疗方法的治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
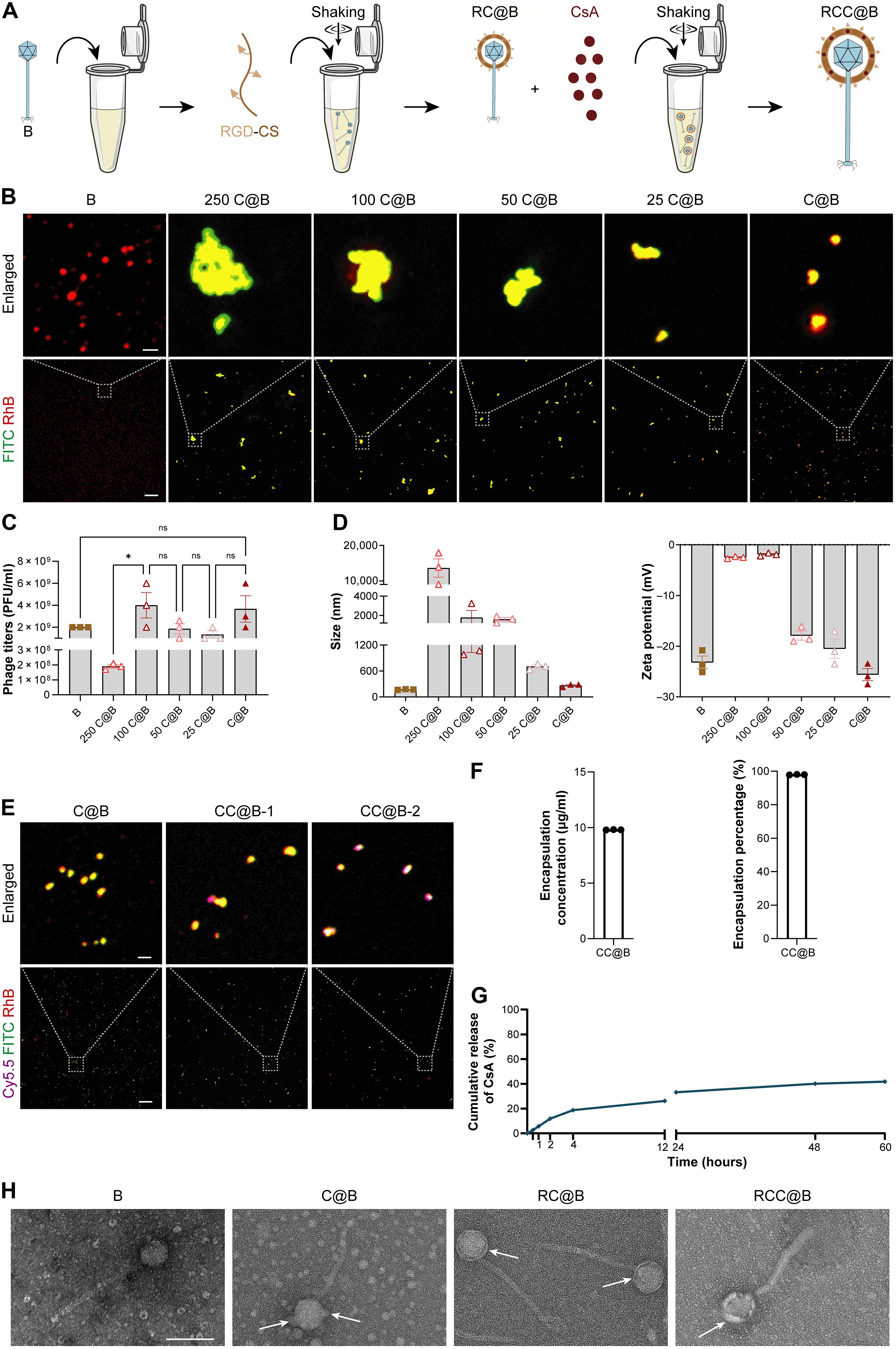
Wearing bacteriophages individually with an adhesive drug-loadable nanohelmet for treating ocular infections
Ocular infections pose notable blindness risks. Despite its advantages in inhibiting multidrug-resistant bacteria and eliminating biofilms, phage therapy suffers low phage vitality, limited ocular retention, and lack of anti-inflammatory abilities toward ocular infections. Here, wearing phages individually with an adhesive drug-loadable nanohelmet is reported to advance phage therapy. The nanohelmet is formed by depositing arginine-glycine-aspartic acid–modified chitosan on negatively charged phage head through electrostatic interactions, followed by codepositing anti-inflammatory agents via physical adsorption. Nanohelmet formation shows a negligible influence on phage vitality and is applicable to helmet diverse phages. Because of the cationic nature and the presence of arginine-glycine-aspartic acid sequence, such nanohelmet exhibits a double-lock adhesion fashion to ocular surface, prolonging phage retention after instillation. Together with sustained drug release, helmeted phages potently inhibit bacteria, eliminate biofilms, and effectively suppress localized inflammation. In mice with multidrug-resistant pathogen-induced keratitis, helmeted phages achieve superior therapeutic efficacies, even compared to clinical therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


