动态共价脂质纳米颗粒介导抗小鼠脉络膜新生血管的CRISPR-Cas9基因组编辑
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
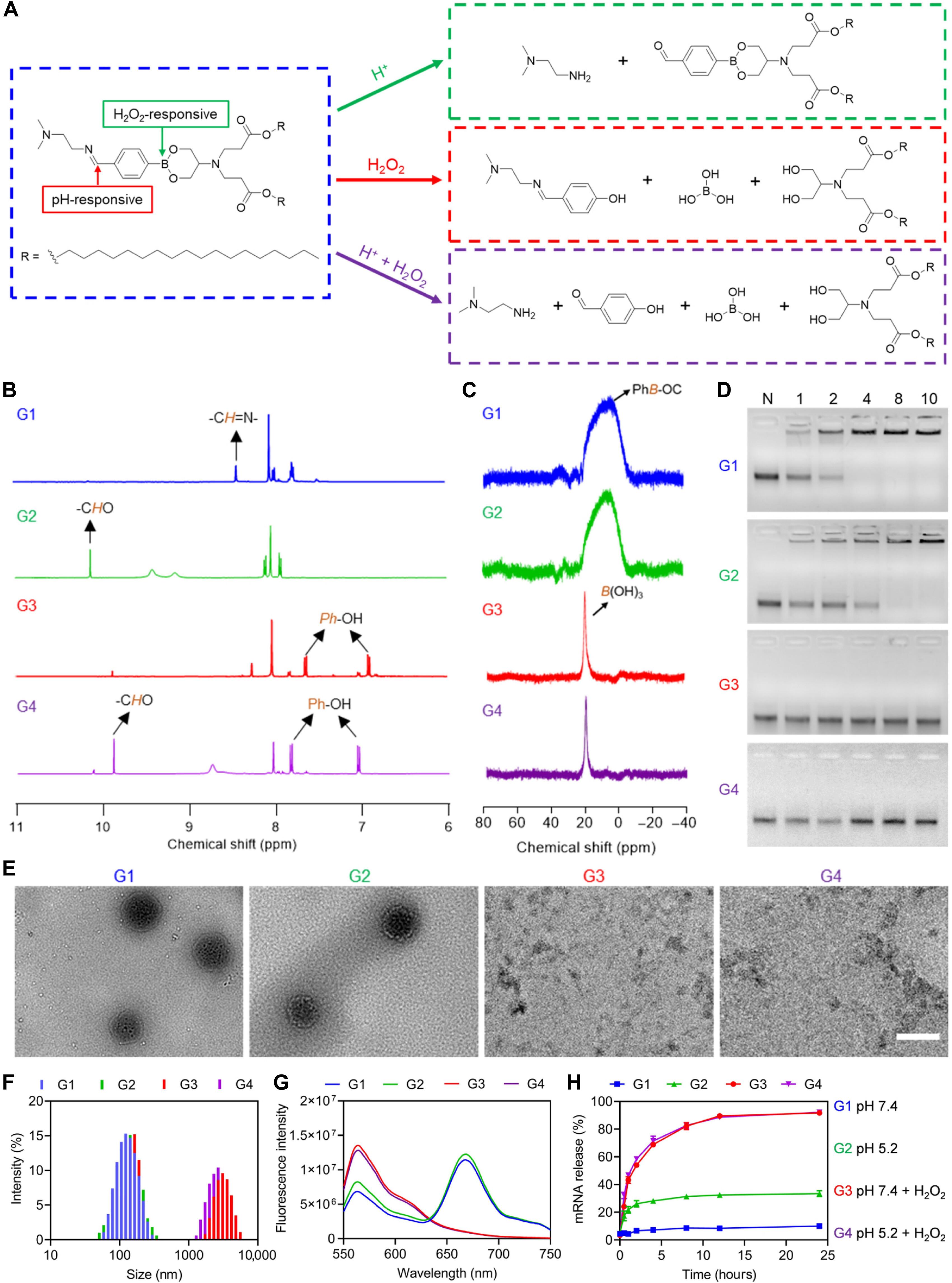
作为脉络膜新生血管(CNV)治疗的一种重要方式,玻璃体内注射血管内皮生长因子A (VEGFA)抑制剂存在不良反应率、患者依从性低和眼部损伤等问题。在这里,动态共价脂质纳米颗粒(LNPs)通过共同递送靶向VEGFA (sgVEGFA)的Cas9 mRNA (mCas9)和单导RNA (sgRNA)来介导VEGFA基因编辑和CNV处理。通过简便的“一锅法”合成,建立了具有亚氨基硼酸酯连锁的脂质文库,并将性能最好的脂质A - 4b3c7配成mRNA转染效率最高的LNP-A - 4b3c7。在患病的视网膜色素上皮细胞内,LNPs在h2o2触发的类脂降解中被解离,促进mRNA/sgRNA的释放,从而增强基因编辑效率。在激光诱导的CNV小鼠中,单次玻璃体内注射mCas9/sgVEGFA@LNP-A 4 b3c7可导致明显的VEGFA破坏和CNV面积减少,在诱导持续治疗效果方面优于临床抗vegf药物。该研究为mRNA传递和基因组编辑建立了一个强大的非病毒平台,并为CNV治疗提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamically covalent lipid nanoparticles mediate CRISPR-Cas9 genome editing against choroidal neovascularization in mice
As an important modality for choroidal neovascularization (CNV) treatment, intravitreal injection of vascular endothelial growth factor A (VEGFA) inhibitors suffers from undesired response rate, low patient compliance, and ocular damage. Here, dynamically covalent lipid nanoparticles (LNPs) were engineered to mediate VEGFA gene editing and CNV treatment by codelivering Cas9 mRNA (mCas9) and single guide RNA (sgRNA) targeting VEGFA (sgVEGFA). A library of lipidoids bearing iminoboronate ester linkage was developed via facile “one-pot” synthesis, and the top-performing lipidoid-A4B3C7 was formulated into LNP-A4B3C7 with the highest mRNA transfection efficiency. Inside the diseased retinal pigment epithelial cells, LNPs were dissociated upon H2O2-triggered lipidoid degradation, facilitating mRNA/sgRNA release to potentiate the gene editing efficiency. In laser-induced CNV mice, mCas9/sgVEGFA@LNP-A4B3C7 after single intravitreal injection led to pronounced VEGFA disruption and CNV area reduction, outperforming the clinical anti-VEGF drug in eliciting sustained therapeutic effect. This study establishes a robust nonviral platform for mRNA delivery and genome editing and renders a promising strategy for CNV treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


