硼基聚不对称取代乙醇酸酯纳米微球
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
以非对称取代乙二醇酯(ASG, I-VI)单体(线性烷基(丙基甲基- i和丙基乙基- ii)或支链(异异丁基甲基- iii,异丁基乙基- iv,异亮氨酸甲基- v,异亮氨酸乙基- vi)乙二醇酯)为原料,制备了具有光学活性的硼基非对称结构聚(取代乙二醇酯)均聚物(BF2I-PASG)。在无溶剂环境下,在Sn(Oct)2催化剂存在下,以BF2dbmOEtOH (BF2I)为引发剂。DSC分析表明,非晶BF2I-PASG (Tg: 4.1-31.9°C)的存在适合于短期药物释放,这是由于单体I-VI的不对称结构,包括GA环上的各种取代侧基。通过TGA分析检测了BF2I-PASG的热稳定性(即Td),表明随着聚合物主链上烷基侧基长度的增加,Td值从346℃转移到377℃,这是一个单步降解过程。BF2I-PASGs的消光系数高达53750 M -⁻¹·cm -⁻,表现出强烈的π-π*跃迁和强烈的蓝色荧光,单指数衰减后的荧光寿命高达1.96 ns,量子产率高达75%。此外,这些聚合物的纳米颗粒(NPs)具有光滑的表面和规则的球形,平均直径小至236 nm,低而窄的多分散指数(PDI)在0.062 ~ 0.136之间,载药量约为≥5%,显示出紫杉醇抗癌药物的控释特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
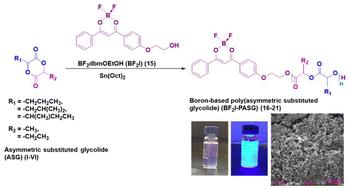
Boron-based poly(asymmetric substituted glycolide) nanospheres†
Boron-based asymmetrically structured poly(substituted glycolide) homopolymers (BF2I-PASG) were prepared from a library of asymmetric substituted glycolide (ASG, ) monomers (linear alkyl (propyl methyl- and propyl ethyl-) or branched (isobutyl methyl-, isobutyl ethyl-, isoleucine methyl-, and isoleucine ethyl-) glycolide), which have an analogous structure to lactide (LA) and glycolide (GA), using BF2dbmOEtOH (BF2I) as the initiator in the presence of the Sn(Oct)2 catalyst in a solvent-free environment. The presence of amorphous BF2I-PASG, with a glass transition temperature (Tg) ranging from 4.1 to 31.9 °C determined by DSC analysis, suggested its suitability for short-term drug release. This amorphous character is attributed to the asymmetric structure of monomers , which incorporate various substituted side groups on the GA rings. The thermal stability (i.e., Td) of BF2I-PASG was examined by TGA analysis, revealing a single-step degradation that shifted towards higher Td values, from 346 °C to 377 °C, as the length of the alkyl side group on the polymer backbone increased. BF2I-PASGs exhibit high extinction coefficients of up to 53 750 M−1 cm−1, demonstrating strong π–π* transitions and intense blue fluorescence, with fluorescence lifetimes following a single-exponential decay as high as 1.96 ns and good quantum yields of up to 75%. Moreover, the nanoparticles (NPs) of these polymers possess a smooth surface and regular spherical shape, with average diameters as small as 236 nm, low and narrow polydispersity indices (PDI) ranging from 0.062 to 0.136, and a drug loading capacity of approximately ≥5%. For example, the NPs exhibited a rapid release profile for the anticancer drug paclitaxel, with around 82.1–96.2 of the drug released within approximately 5 days, depending on the NP composition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


