铁掺杂和阴离子保护的协同策略实现了高效和稳健的海水电解
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
海水电解是一种很有前途的制氢方法,但产生腐蚀性氯(如氯化物和次氯酸盐)仍然是一个关键的挑战。本文通过Fe掺杂和NiMoO4/NF的后续磷化,成功合成了FeNiP/MoOx/NiMoO4/NF电催化剂,该催化剂对碱性海水氧化具有优异的活性和稳定性。FeNiP/MoOx/NiMoO4/NF需要349 mV的过电位才能达到1000 mA cm−2的电流密度(j),在相同的电流密度下可连续工作500 h。此外,配备FeNiP/MoOx/NiMoO4/NF的阴离子交换膜电解槽表现出优异的性能,仅需2.12 V即可达到300 mA cm−2,并保持180 h的稳定运行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
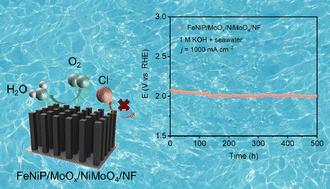
A synergistic strategy of Fe doping and anion protection enables efficient and robust seawater electrolysis†
Seawater electrolysis is a promising method for producing hydrogen, but the generation of corrosive chlorine species (e.g., chloride and hypochlorite) at anodes remains a critical challenge. Herein, with the use of Ni foam (NF) as a catalyst support, we developed a FeNiP/MoOx/NiMoO4/NF as the anode for alkaline seawater oxidation. The incorporation of Fe enhances charge transfer and promotes the generation of active sites, and the in situ generated PO43− and MoO42− species effectively repel Cl−, thereby significantly enhancing the electrode's corrosion resistance. This electrode demands a low overpotential of 349 mV to drive 1000 mA cm−2 and is capable of continuous operation for 500 h in alkaline seawater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


