微生物暗物质引领了埃及塔巴太阳湖的生物地球化学循环
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
微生物暗物质(MDM)代表了大量未培养的微生物生命,在很大程度上具有未知的生态作用,特别是在极端环境中。本研究利用散枪宏基因组学(~ 70 M reads/sample)研究了埃及塔巴太阳湖高盐微生物垫中的MDM;在四个地点进行三次抽样)。总共回收了364个宏基因组组装基因组(MAGs),其中116个(约30%)被归类为MDM,包括55%的古细菌谱系和45%的细菌谱系。功能注释显示,约14%的MDM mag具有固定碳的遗传潜力。Ca. Lokiarchaeota(5个MAG, 4.3%)和Heimdallarchaeota(1个MAG, 0.96%)的基因组成表明它们是一种混合营养的生活方式。一些非产甲烷mag具有利用甲醇和甘氨酸-甜菜碱作为碳源的遗传能力。此外,Ca. Marinisomatota MAGs具有降解多糖的遗传潜力,而KSB1 MAGs具有碳水化合物降解、反硝化和固氮的基因。Ca. asgardarchaaeota和Ca. Coatesbacteria (rtg -13 - 66 - 14)中SOX基因复合物的相对丰度较高,这表明MDM群落参与硫代硫酸盐氧化。此外,一种新的粘球菌MAG编码了一个完整的光合基因簇,包括光系统II,表明与蓝藻一样具有光养活性。总的来说,太阳湖MDM群落的基因组成支撑了碳循环、硫还原、硫代硫酸盐氧化、固氮和反硝化等关键过程,推动了这个独特的高盐生态系统的生物地球化学动力学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
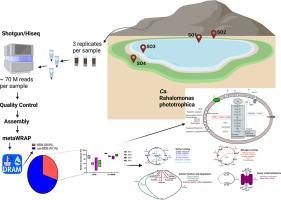
Microbial dark matter spearheading the biogeochemical cycle in the Solar Lake of Taba, Egypt
Microbial dark matter (MDM) represents a vast, uncultured fraction of microbial life with largely unknown ecological roles, particularly in extreme environments. This study investigates MDM in the hypersaline microbial mats of Solar Lake, Taba, Egypt, using shotgun metagenomics (∼70 M reads/sample; triplicate sampling across four sites). A total of 364 metagenome-assembled genomes (MAGs) were recovered, of which 116 (∼30 %) were classified as MDM, comprising 55 % archaeal and 45 % bacterial lineages. Functional annotation revealed that ∼14 % of the MDM MAGs had the genetic potential to fix carbon. The genetic makeup of Ca. Lokiarchaeota (5 MAGs, 4.3 %) and Heimdallarchaeota (1 MAG, 0.86 %) suggest a mixotrophic lifestyle. Some non-methanogenic MAGs had the genetic capacity to utilize methanol and glycine-betaine as carbon sources. In addition, Ca. Marinisomatota MAGs had the genetic potential to degrade polysaccharides, while KSB1 MAGs harbored genes for carbohydrate degradation, denitrification, and nitrogen fixation. The high relative abundance of the SOX gene complex, in Ca. Asgardarchaeota and Ca. Coatesbacteria (RBG-13–66–14), highlights MDM community involvment in thiosulfate oxidation. Additionally, a novel Myxococcota MAG encoded a complete photosynthetic gene cluster, including photosystem II, suggesting phototrophic activity along with Cyanobacteria. Collectively, the genetic makeup of the Solar Lake MDM community underpins key processes such as carbon cycling, sulfur reduction, thiosulfate oxidation, nitrogen fixation, and denitrification, driving the biogeochemical dynamics of this unique hypersaline ecosystem.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


