利用新疆褐藻的新型还原酶AxSDR高效合成(R)-1-(3-(三氟甲基)苯基)乙醇
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
(R)-1-(3-(三氟甲基)苯基)乙醇((R)-1b)是合成(R)-3-(1-(3-(三氟甲基)苯基)乙氧基)氮杂丁-1-羧酰胺等神经保护药物的重要手性中间体。本研究通过基因挖掘策略,从新疆褐藻(Algoriella xinjiangensis, AxSDR)中发现了一种新的短链脱氢酶/还原酶。还原酶AxSDR表现出出色的还原能力,并在大肠杆菌BL21(DE3)中成功表达。表达AxSDR的重组大肠杆菌对前手性芳香酮具有不同的催化活性。值得注意的是,与邻位取代的对应物相比,AxSDR对取代在间位或对位的酮具有强烈的偏好。通过同源性建模和分子对接来阐明AxSDR对底物选择性的分子基础。此外,通过优化反应参数,建立了将3′-(三氟甲基)苯乙酮(1a)转化为(R)-1b的高效非对称生物还原工艺,以表达AxSDR的重组大肠杆菌全细胞为生物催化剂,将1500 mmol·L-1 1a转化为(R)-1b,优化条件下产率为99.4%,对映体超额率为99.8%,时空产率(STY)为523 g·L-1·d-1。这些结果代表了迄今为止报道的(R)-1b生产中最高的基板载荷和STY。值得注意的是,AxSDR是第一个从新疆紫杉中报道的具有还原活性的还原酶,其优异的性能突出了其作为工业规模制备(R)-1b生物催化剂的潜力本文章由计算机程序翻译,如有差异,请以英文原文为准。
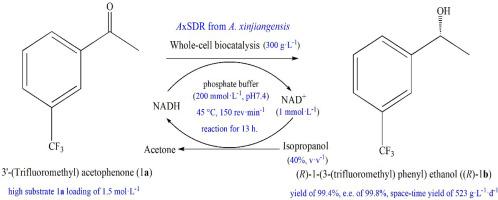
Efficient biosynthesis of (R)-1-(3-(trifluoromethyl)phenyl)ethanol using a novel reductase AxSDR from Algoriella xinjiangensis
(R)-1-(3-(trifluoromethyl)phenyl)ethanol ((R)-1b) is a crucial chiral intermediate for synthesizing neuroprotective drugs, such as (R)-3-(1-(3-(trifluoromethyl)phenyl)ethoxy) azetidine-1-carboxamide. In this study, through gene mining strategies, a novel short-chain dehydrogenase/reductase from Algoriella xinjiangensis (AxSDR) was discovered. Reductase AxSDR exhibits outstanding reduction capabilities, and was successfully expressed in Escherichia coli BL21(DE3). The recombinant E. coli expressing AxSDR demonstrated differential catalytic activities toward prochiral aromatic ketones. Notably, AxSDR have strong preference for ketones substituted at the meta- or para-positions, as opposed to their ortho-substituted counterparts. Homology modeling and molecular docking were performed to elucidate the molecular basis of AxSDR's substrate selectivity. Furthermore, by optimizing reactions parameters, a highly efficient asymmetric bioreduction process was established for converting 3′-(trifluoromethyl)acetophenone (1a) to (R)-1b Using recombinant E. coli whole cells expressing AxSDR as biocatalysts, 1500 mmol·L-1 1a was converted to (R)-1b, achieving a yield of 99.4 %, an enantiomeric excess of 99.8 % and a space-time yield (STY) of 523 g·L-1·d-1 under optimized conditions. These results represent the highest substrate loading and STY reported to date for (R)-1b production. Significantly, the AxSDR is the first reductase with reducing activity reported from A. xinjiangensis, and its exceptional performance highlights its potential as a promising biocatalyst for the industrial-scale preparation of (R)-1b
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


