锰(III)介导的二芳基膦氧化物和四硫化物的化学选择性自由基偶联:二硫代磷酸盐和硫代磷酸盐的合成。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种高效的二硫代磷酸盐合成策略,其中二苯基膦氧化物在Mn(III)氧化下与四硫化物发生化学选择性自由基反应。由于四硫化物的位阻作用,这一过程产生了各种功能性二硫代磷酸盐和具有高化学选择性的磷酸盐。该反应简单,使用现成的材料,并具有良好的兼容性,使其在进一步应用中具有很高的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
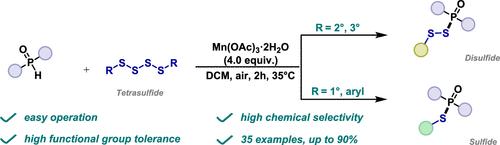
Manganese(III)-Mediated Chemoselective Radical Coupling of Diarylphosphine Oxides and Tetrasulfides: Synthesis of Dithiophosphates and Thiophosphates
We developed an efficient synthetic strategy for dithiophosphates, where diphenylphosphine oxides undergo chemoselective radical reactions with tetrasulfides under Mn(III) oxidation. This process produces various functional dithiophosphates and phosphates with high chemical selectivity, attributed to the steric hindrance of the tetrasulfides. The reaction is simple, uses readily available materials, and offers excellent compatibility, making it highly practical for further applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


