从羧酸或其衍生物到胺和醚:可持续形成C-N和C-O键的现代脱羧方法。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
胺和醚是药物、天然产物、有机材料和催化系统中必不可少的结构基序。因此,开发新颖、环保、经济的碳氮键和碳氧键构建策略对这些化合物的合成具有重要意义。近年来,羧酸及其衍生物已成为有吸引力的、廉价的、无毒的、容易获得的合成基石,作为芳基卤化物的有希望的替代品。越来越多的证据表明,羧酸衍生物的脱羧胺化和醚化为胺和醚的合成提供了一种强有力的方法。这些转变通过三个主要的机制途径进行,每个途径都提供高原子经济性。具体来说,通过杂解脱羧产生的碳离子(或有机金属物质)可以与合适的亲电试剂反应形成c -杂原子键。相反,通过均溶脱羧产生的以碳为中心的自由基可以通过自由基重组或氧化俘获与杂原子基试剂偶联。此外,碳正离子通常是通过羧酸的电化学氧化形成的:氧化脱羧首先产生碳自由基,然后在阳极进一步氧化生成碳正离子。这种高度亲电的中间体随后可以被杂原子亲核试剂截获,形成C-N或C-O键。本文综述了该领域的最新进展,重点介绍了过渡金属催化、光氧化还原催化以及脱羧胺化和醚化的电化学方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
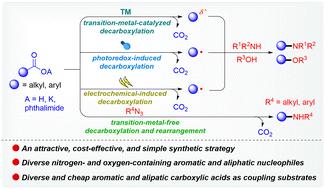
From carboxylic acids or their derivatives to amines and ethers: modern decarboxylative approaches for sustainable C–N and C–O bond formation
Amines and ethers represent essential structural motifs in pharmaceuticals, natural products, organic materials, and catalytic systems. The development of novel, environmentally friendly, and cost-effective strategies for constructing C–N and C–O bonds is therefore of significant importance for the synthesis of these compounds. In recent years, carboxylic acids and their derivatives have emerged as attractive, inexpensive, non-toxic, and readily available synthetic building blocks, serving as promising alternatives to aryl halides. Growing evidence has demonstrated that decarboxylative amination and etherification of carboxylic acid derivatives offer a powerful approach for the synthesis of amines and ethers. These transformations proceed via three principal mechanistic pathways, each offering high atom economy. Specifically, carbanions (or organometallic species) generated through heterolytic decarboxylation can react with suitable electrophiles to form C–heteroatom bonds. In contrast, carbon-centred radicals produced through homolytic decarboxylation can couple with heteroatom-based reagents via radical recombination or oxidative trapping. Additionally, carbocations are typically formed via electrochemical oxidation of carboxylic acids: oxidative decarboxylation first yields a carbon radical, which is then further oxidized at the anode to generate a carbocation. This highly electrophilic intermediate can subsequently be intercepted by heteroatom nucleophiles to construct C–N or C–O bonds. This review highlights recent advances in the field, with a focus on transition metal catalysis, photoredox catalysis, and electrochemical methods for decarboxylative amination and etherification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


