双金属磷酸盐:活性和稳定的碱性整体水分解双功能电催化剂
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氢(H2)能源被认为是一种不排放温室气体的有前途的能源。在各种技术中,通过电化学水分解(EWS)技术可以经济高效地生产这种能量。本研究通过水化学生长法合成了多种双金属磷酸盐,特别是磷酸钴、磷酸钴镍、磷酸钴铜和磷酸钴铁(CIP)。通过x射线衍射(XRD)、扫描电子显微镜(SEM)、能谱(EDS)和傅里叶变换红外光谱(FTIR)对合成的双金属磷酸盐进行了物理表征,以评价其结晶度、形貌、组成和表面功能特征。并对所制备的电催化剂进行了电化学研究,以确定其双功能电化学性能。其中,CIP是一种具有析氢反应(HER)和析氧反应(OER)双功能活性的优化电催化剂。有趣的是,在1.0 M氢氧化钾(KOH)介质中,当电流密度为10 mA cm - 2时,CIP催化剂的HER过电位最低,为325 mV, OER过电位最低,为300 mV。优化后的催化剂具有较低的Tafel斜率值,分别为53.6和58.4 mV.dec−1,具有较快的反应动力学速率。此外,它是稳定和持久的长达40-45小时的HER和OER活动。CIP还增强了从更高的周转频率(TOF)值和规范化数据验证的内在行为。此外,由于电化学活性表面积(ECSA)高达342.5 cm2, H2和O2的理论产率分别为470.6 × 10−6和44.5 × 10−6 mol s−1 cm−1。这种新型的双金属(Co -Fe)磷酸基电催化剂在实际应用中可作为PGM金属基电催化剂的替代材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
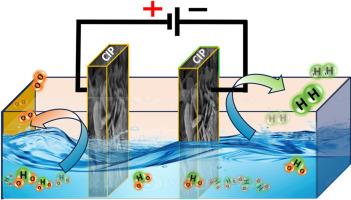
Bi-metallic phosphate: Active and stable bifunctional electrocatalysts for alkaline overall water splitting
Hydrogen (H2) energy is considered as promising energy with no emission of greenhouse gases. This energy can be produced economically and efficiently via electrochemical water splitting (EWS) technique among various other techniques. Herein, this study comprises the synthesis of various bi-metallic phosphates, particularly cobalt phosphate, cobalt nickel phosphate, cobalt copper phosphate, and cobalt iron phosphate (CIP) through aqueous chemical growth method. As-synthesized bi-metallic phosphates have been explored for physical characterization through X-ray diffraction (XRD), Scanning electron microscopy (SEM), Energy dispersive spectroscopy (EDS), and Fourier transform infrared spectroscopy (FTIR) to evaluate their crystallinity, morphology, composition, and surface functionality features. The electrochemical investigation is also carried out to determine bifunctional electrochemical performance of prepared electrocatalysts. Wherein, CIP was found as an optimized electrocatalyst for bi-functional activity towards hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Interestingly, the CIP catalyst exhibits the lowest overpotential of 325 mV for HER and 300 mV for OER at 10 mA cm−2 current density in 1.0 M potassium hydroxide (KOH) media. The optimized catalyst offers a fast rate of reaction kinetics by revealing lower Tafel slope values i.e., 53.6 and 58.4 mV.dec−1, respectively. In addition, it is stable and durable up to 40–45 h for HER and OER activity. The CIP has also enhanced intrinsic behavior validated from higher Turnover frequency (TOF) values and normalized data. Moreover, high theoretical H2 and O2 production rate obtained as 470.6 × 10−6 and 44.5 × 10−6 mol s−1 cm−1 due to large electrochemical active surface area (ECSA) of 342.5 cm2. This novel bi-metallic (Co -Fe) phosphate-based electrocatalyst can be a replaceable materials for the PGM metal based electrocatalysts practically.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


