碱金属修饰B4C3增强储氢:通往高容量储氢的途径
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
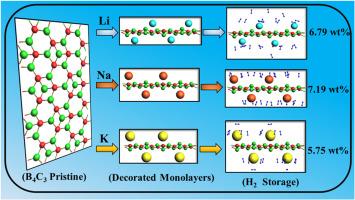
实现氢经济取决于开发负担得起的高性能储氢材料。本文报道了基于第一性原理计算的碱金属原子Li、Na和K修饰B4C3单层的储氢前景。计算结果表明,B4C3单层具有坚固且微屈曲的类石墨烯结构(直接带隙为1.91 eV),是一种极好的储氢基质。通过诱导电荷极化,原始单层能够物理吸附多达9个氢分子,平均吸附能为- 0.09 eV,实现2.77 wt%的重力密度。用碱金属(Li, Na, K)对B4C3进行修饰,使单层表面的金属原子成键,其结合能在−2.17 ~−2.50 eV之间,且未发生团聚。装饰明显提高了材料的H2吸附能力,物理吸附能在−0.42 ~−0.58 eV之间。Li@B4C3和Na@B4C3可吸附多达5个氢分子,而K@B4C3在最佳吸附能(−0.10 ~−0.60 eV)下可在可逆物理吸附下存储6个氢分子,且没有明显的结构畸变。四分之一的金属覆盖单层具有显著的高密度,分别为6.79 wt% (4Li@B4C3)、7.19 wt% (4Na@B4C3)和5.75 wt% (4K@B4C3),解吸温度从185 K到355 K (1atm),表明该材料具有实际应用价值。负载氢的装饰单层在室温下是热稳定的,其中na装饰板的重量密度为7.19 wt%,并且具有可逆储氢的实际解吸温度,击败了几种当代材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced hydrogen storage in alkali metal-decorated B4C3: A pathway towards high-capacity hydrogen storage
Realizing a hydrogen-based economy hinges on the development of affordable and high-performance hydrogen storage materials. This work reports hydrogen storage prospects of alkali metal atoms Li, Na and K decorated B4C3 monolayers based on first-principles calculations. The calculations reveal that the B4C3 monolayer, demonstrating robust and slightly buckled graphene-like structure (a direct bandgap of 1.91 eV), is an excellent matrix to store hydrogen. The pristine monolayer is capable to physiosorb up to 9 hydrogen molecules with an average adsorption energy of −0.09 eV, realizing a gravimetric density of 2.77 wt%, via induced charge polarization. The decoration of B4C3 with alkali metals (Li, Na, K) was carried out which caused bonding of the metal atoms on the surface of the monolayer with high binding energies ranging from −2.17 to −2.50 eV and without causing agglomeration. The decoration appeared to significantly enhance H2 adsorption capacity of the material offering physisorption energies in range −0.42 to −0.58 eV. Li@B4C3 and Na@B4C3 could adsorb up to 5 whereas K@B4C3 store 6 hydrogen molecules under reversible physisorption at optimal adsorption energies (−0.10 to −0.60 eV) without notable structural distortion. The one-fourth metal covered monolayer exhibited significantly high densities of 6.79 wt% (4Li@B4C3), 7.19 wt% (4Na@B4C3), and 5.75 wt% (4K@B4C3), with desorption temperatures ranging from 185 K to 355 K (at 1 atm) pointing to practical utilization of the materials. The hydrogen-loaded decorated monolayers are thermally stable at room temperature, with the Na-decorated slab indicating gravimetric density of 7.19 wt% accompanied by practical desorption temperatures for reversible hydrogen storage, beating several contemporary materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


