具有抗菌和nets降解双重功能的双纳米酶负载核-壳微针贴剂用于牙周炎治疗
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
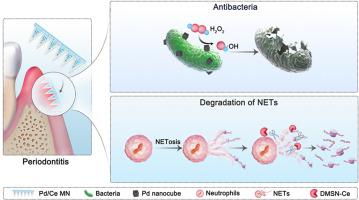
牙周炎是一种慢性炎症性疾病,影响全世界超过10亿人,其特点是细菌感染和免疫反应过度活跃。最近的研究表明,中性粒细胞胞外陷阱(NETs)的形成对牙周组织的破坏有重要作用,而NETs的降解在牙周炎的治疗中起着关键作用。目前的治疗方法,包括机械清创和全身抗生素,面临抗生素耐药性和局部疗效不足等局限性。为了整合抗菌和nets消除策略,作者提出了一种新的治疗方法,使用双功能核-壳微针(MNs)递送两种类型的纳米酶:内心层的过氧化物酶(POD)样钯(Pd)纳米酶和外层的dna样树突状介孔二氧化硅纳米颗粒(DMSN)-铈(Ce)纳米酶。Pd/Ce MNs旨在促进Pd的快速释放以消除细菌,并促进DMSN-Ce的持续释放以降解NETs。本研究详细介绍了两种纳米酶和核壳结构MNs的合成和表征,随后评估了它们的催化活性、体外生物相容性、抗菌功效和net -裂解能力。使用大鼠牙周炎模型的体内测试表明,在细菌清除,炎症减少和牙槽骨保存方面有显着改善。总之,这些研究结果表明,具有优异抗菌和nets水解特性的Pd/Ce MNs是治疗牙周炎的一种很有前景的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual nanozymes-loaded core-shell microneedle patches with antibacterial and NETs-degradation bifunctional properties for periodontitis treatment
Periodontitis, a chronic inflammatory disease affecting over one billion people worldwide, is characterized by bacterial infections and hyperactive immune responses. Recent studies have revealed that the formation of neutrophil extracellular traps (NETs) contributes significantly to periodontal tissue destruction, and NETs degradation plays a critical role in periodontitis treatment. Current treatments, including mechanical debridement and systemic antibiotics, face limitations such as antibiotic resistance and insufficient local efficacy. To integrate antibacterial and NETs-elimination strategies, the authors propose a novel therapeutic approach using bifunctional core-shell microneedles (MNs) that deliver two types of nanozymes: a peroxidase (POD)-like palladium (Pd) nanozyme in the inner core layer and a DNase-like dendritic mesoporous silica nanoparticles (DMSN)-cerium (Ce) nanozyme in the outer layer. The Pd/Ce MNs are designed to facilitate the rapid release of Pd for bacterial eradication and the sustained release of DMSN-Ce for NETs degradation. This study details the synthesis and characterization of two nanozymes and core-shell structured MNs, followed by evaluations of their catalytic activities, in vitro biocompatibility, antibacterial efficacy and NETs-cleavage ability. In vivo testing using a rat model of periodontitis demonstrates significant improvements in bacterial clearance, inflammation reduction, and alveolar bone preservation. In conclusion, these findings suggest that Pd/Ce MNs with superior antibacterial and NETs-hydrolyzing properties represent a promising therapeutic strategy for the management of periodontitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


