Li6.5La3Zr1.5Ta0.5O12| Li2.25Zr0.75Fe0.25Cl6异质电解质界面低压接触力学及电化学性能
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
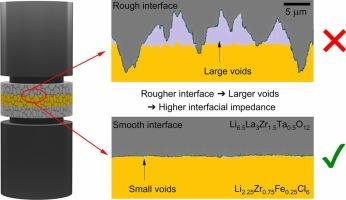
虽然有许多固体电解质(SE),但没有一种同时满足锂(Li)金属固态电池实际应用的所有标准。多层电解质结构引起了人们的极大关注,其中一种电解质与阳极接触,另一种用作阴极,在两个se之间形成异质电解质界面。异质电解质界面的研究,特别是在低压下(<;5mpa),是有限的。本文研究了高压稳定卤化物Li2.25Zr0.75Fe0.25Cl6 (LZFC)与与Li稳定的氧化物Li6.5La3Zr1.5Ta0.5O12 (LLZTO)之间异质电解质界面的接触力学和界面电阻。我们报告了任一SE的表面粗糙度强烈影响LLZTO|LZFC界面电阻(Rint)在5 MPa以下。通过控制界面粗糙度,将Rint从3280 Ωcm2降低到648 Ωcm2,降低了5倍。我们证明,在80°C下对LLZTO|LZFC界面进行热压可以促进LZFC的蠕变,从而有助于在低压下形成更多的接触,从而使Rint降低50%。此外,用HCl或H3PO4酸洗LLZTO会增加2-6倍的粗糙度,并导致HCl处理的LLZTO与LZFC之间的界面不稳定。然而,在热压后,一个LLZTO|LZFC|LLZTO对称电池循环50次,每半个循环通过1 mAh/cm2的电荷,无论LLZTO表面处理如何,电阻都没有增加,表明钝化了LLZTO|LZFC界面。这项工作为低堆压下多层ssb的设计和构造提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Contact mechanics and electrochemical properties of the Li6.5La3Zr1.5Ta0.5O12| Li2.25Zr0.75Fe0.25Cl6 hetero-electrolyte interface in a low-pressure regime
While there are numerous solid electrolytes (SE), not one simultaneously meets all the criteria to enable practical application of lithium (Li) metal solid-state batteries. The multilayer electrolyte configuration has garnered significant attention where one electrolyte is used in contact with the anode and another one as a catholyte, creating a hetero-electrolyte interface between the two SEs. Studies of hetero-electrolyte interfaces, especially at low pressures (< 5 MPa), are limited. This work investigates the contact mechanics and interfacial resistance of the hetero-electrolyte interface between the high voltage stable halide Li2.25Zr0.75Fe0.25Cl6 (LZFC) and the oxide Li6.5La3Zr1.5Ta0.5O12 (LLZTO), which is stable with Li. We report that the surface roughness of either SE strongly affects the LLZTO|LZFC interfacial resistance (Rint) below 5 MPa. By controlling the interface roughness, Rint was decreased by a factor of 5 – from 3280 Ωcm2 to 648 Ωcm2. We demonstrate that warm pressing of the LLZTO|LZFC interface at 80 °C promotes creep of the LZFC to assist in forming more contact at low pressures, such that Rint decreased by 50 %. Furthermore, acid washing the LLZTO with HCl or H3PO4 imparts additional roughness by 2-6× and leads to an interfacial instability between HCl treated LLZTO and LZFC. Nevertheless, after warm pressing, a LLZTO|LZFC|LLZTO symmetric cell was cycled for 50 cycles, passing 1 mAh/cm2 of charge per half cycle with no increase in resistance regardless of the LLZTO surface treatment, indicating a passivated LLZTO|LZFC interface. This work provides insight into the design and construction of multilayer SSBs at low stack pressures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


