用于增材制造的光降解基序
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
我们引入了三氧烷作为一种新的基序,通过使用光酸发生器(PAGs)的正交光源,按需光解3D打印聚合物网络。酸诱导降解非常稳定的三氧杂环,再加上生成含三氧杂环单体的多功能性,使得该基序特别有趣。在这里,我们展示了使用基序作为三功能烯交联剂与三功能脂环硫醇的组合。因此,将潜伏PAG (UVI 6976)纳入光聚合制剂中,并证明了其在较长波长的光固化期间的潜伏期。光聚合后,含有PAG的材料表现出与不含PAG的参考网络相同的(热)机械性能。在紫外光照射下,通过核磁共振,DSC和溶解度测试证明了三氧烷的降解。最后,证明了含三氧环的配方易于印刷。本文章由计算机程序翻译,如有差异,请以英文原文为准。
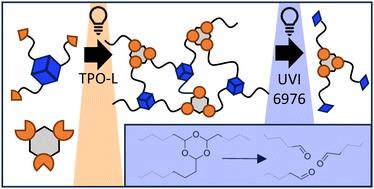
Trioxanes as photodegradable motifs for additive manufacturing†
We introduce trioxanes as a new motif for on-demand photolysis of 3D printed polymer networks via an orthogonal light source using photoacid generators (PAGs). The acid-induced degradation of otherwise very stable trioxanes in combination with the versatility of generating trioxane-containing monomers makes the motif particularly interesting. Herein, we demonstrate the use of the motif as a trifunctional ene-crosslinker in combination with a trifunctional alicyclic thiol. Thereby, a latent PAG (UVI 6976, absorption ≤ 400 nm) is included into the photopolymerizable formulation and its latency during photocuring at longer wavelengths (≥400 nm) is proven. Upon photopolymerization, the PAG-containing material exhibits equal (thermo)mechanical performance to a reference network excluding PAG. Upon UV light irradiation, degradation of trixoanes is proven via NMR, DSC and solubility tests. Finally, facile printing of the trioxane-containing formulation has been shown.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


