快速去除PFOA的介孔二氧化硅纳米颗粒:表面官能团对吸附效率和吸附剂再生的影响
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
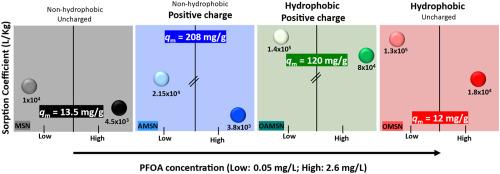
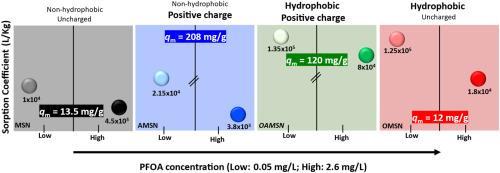
本研究探索了通过油水两相分层合成介孔二氧化硅纳米颗粒(MSN),并与正辛基三氯硅烷(OTS)、3-氨基丙基三乙氧基硅烷(APTES)或两者都功能化,得到OMSN、AMSN和OAMSN。所有吸附剂都能快速去除水中的全氟辛酸(PFOA), 10分钟内达到95%的吸附平衡。吸附动力学结果表明,OAMSN的准二级速率常数最高,其孔径最大(14.4 nm)。等温线分析表明,在较宽的平衡浓度范围内(Ce: 0.2 μg/L ~ 8 mg/L), OAMSN和AMSN均具有较高的平衡负荷,覆盖了与环境相关的PFOA浓度。表面带正电荷的AMSN(零电荷点= 9)表现出最大的吸附量(qm = 208 mg/g),而表面带正电荷的OAMSN在Ce = 50 μg/L时具有较高的吸附系数(Kd = 1.4 × 105 L/kg)。在Ce = 50 μg/L时,OMSN的Kd值最高,但qm值最低。尽管孔隙体积相似,但孔隙填充计算结果显示差异很大(MSN为0.8%,AMSN为12%),这凸显了表面化学对孔隙结构的主导作用。疏水性决定了低负荷下的吸附,而静电相互作用成为接近qm的关键。溶剂萃取保留了疏水吸附剂(OMSN, OAMSN)的吸附效率,而紫外线活化的过硫酸盐对负载PFOA的降解率高达87%,但影响了重复使用,特别是对功能化材料。因此,溶剂萃取适用于功能化吸附剂,而紫外线/过硫酸盐更适合于非功能化吸附剂的再生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mesoporous silica nanoparticles for rapid removal of PFOA: Impact of surface functional groups on adsorption efficiency and adsorbent regeneration
This study explores mesoporous silica nanoparticles (MSN) synthesized via oil-water biphase stratification and functionalized with n-octyltrichlorosilane (OTS), 3-aminopropyltriethoxysilane (APTES), or both, yielding OMSN, AMSN, and OAMSN. All adsorbents rapidly removed perfluorooctanoic acid (PFOA) from water, reaching 95 % adsorption equilibrium within 10 min. PFOA adsorption kinetics showed that OAMSN, with the largest pore size (14.4 nm), had the highest pseudo-second-order rate constant. Isotherm analysis indicated that both OAMSN and AMSN achieved higher equilibrium loadings across a broad equilibrium concentration range (Ce: 0.2 μg/L - 8 mg/L), which covers environmentally relevant concentration of PFOA. AMSN, with a positively charged surface (point of zero charge = 9), showed the highest maximum adsorption capacity (qm = 208 mg/g), while OAMSN, combining hydrophobicity and positive charge, achieved a much higher adsorption coefficient (Kd = 1.4 × 105 L/kg at Ce = 50 μg/L). OMSN, the most hydrophobic evaluated by water contact angle measurements, had a high Kd at Ce = 50 μg/L but the lowest qm. Pore filling calculations showed large variations (0.8 % for MSN to 12 % for AMSN) despite similar pore volumes, highlighting the dominant role of surface chemistry over pore structure. Hydrophobicity governs adsorption at low loadings, while electrostatic interactions become key approaching qm. Solvent extraction preserved adsorption efficiency in hydrophobic adsorbents (OMSN, OAMSN), while UV-activated persulfate degraded up to 87 % of the loaded PFOA but impaired reuse, especially for functionalized materials. Thus, solvent extraction suits functionalized adsorbents, whereas UV/persulfate is better for regenerating non-functionalized ones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


