3D生物打印神经祖细胞构建增强离体脑整合和神经元-星形胶质细胞分化
Q1 Computer Science
引用次数: 0
摘要
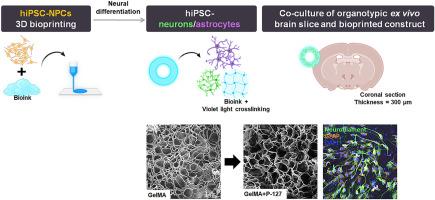
来源于人诱导多能干细胞(hiPSCs)的神经祖细胞(npc)由于其自我更新和分化的潜力,在神经组织工程、疾病建模和再生治疗方面具有很大的前景。在这项研究中,我们利用3D挤出生物打印技术将hipsc - npc封装在由明胶甲基丙烯酰(GelMA)和Pluronic F127 (P-127)组成的复合生物墨水中。这种复合材料被设计成增强基质重塑、机械可调性和细胞特异性分化。结合P-127改善凝胶性、可打印性、肿胀行为、降解动力学和微观结构特征,共同支持增强鼻咽癌增殖。力学特性显示可调节的刚度(杨氏模量:1-8 kPa), GelMA/P-127共混物比单独GelMA表现出更高的强度。免疫染色显示GFAP升高,神经丝M (NeuF-M)表达降低,表明在基质力学的影响下向星形细胞分化转变。钙成像和瞬态信号分析证实了分化神经元的功能活性。基因表达谱支持这些发现,显示GFAP、TUBB3和MAP2上调,SOX2下调,标志着从祖神经表型向成熟神经表型的转变。此外,生物打印构建体与离体脑切片结合,表达TUBB3和NeuF-M,证实了神经元分化能力。这些结果强调了GelMA/P-127复合生物墨水作为工程3D神经组织构建的仿生、可调平台的潜力,为研究神经发育和推进转化再生策略提供了一种多功能工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
3D bioprinted neural progenitor cell constructs for enhancing ex vivo brain integration and neuron-astrocyte differentiation
Neural progenitor cells (NPCs) derived from human induced pluripotent stem cells (hiPSCs) hold great promise for neural tissue engineering, disease modeling, and regenerative therapies due to their self-renewal and differentiation potential. In this study, we utilized 3D extrusion bioprinting to encapsulate hiPSC-NPCs within a composite bioink composed of Gelatin methacryloyl (GelMA) and Pluronic F127 (P-127). This composite was engineered to enhance matrix remodeling, mechanical tunability, and cell-specific differentiation. Incorporating P-127 improved gelation, printability, swelling behavior, degradation kinetics, and microstructural features, collectively supporting enhanced NPC proliferation. Mechanical characterization revealed adjustable stiffness (Young's modulus: 1–8 kPa), with GelMA/P-127 blends exhibiting greater strength than GelMA alone. Immunostaining showed elevated GFAP and reduced Neurofilament M (NeuF-M) expression, indicating a shift toward astrocytic differentiation influenced by matrix mechanics. Calcium imaging and transient signal analysis confirmed the functional activity of the differentiated neurons. Gene expression profiling supported these findings, showing upregulation of GFAP, TUBB3, and MAP2 and downregulation of SOX2, marking the transition from progenitor to mature neural phenotypes. Furthermore, bioprinted constructs integrated with ex vivo brain slices and expressed TUBB3 and NeuF-M, confirming neuronal differentiation capacity. These results underscore the potential of GelMA/P-127 composite bioinks as biomimetic, tunable platforms for engineering 3D neural tissue constructs, offering a versatile tool for studying neurodevelopment and advancing translational regenerative strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioprinting
Computer Science-Computer Science Applications
CiteScore
11.50
自引率
0.00%
发文量
72
审稿时长
68 days
期刊介绍:
Bioprinting is a broad-spectrum, multidisciplinary journal that covers all aspects of 3D fabrication technology involving biological tissues, organs and cells for medical and biotechnology applications. Topics covered include nanomaterials, biomaterials, scaffolds, 3D printing technology, imaging and CAD/CAM software and hardware, post-printing bioreactor maturation, cell and biological factor patterning, biofabrication, tissue engineering and other applications of 3D bioprinting technology. Bioprinting publishes research reports describing novel results with high clinical significance in all areas of 3D bioprinting research. Bioprinting issues contain a wide variety of review and analysis articles covering topics relevant to 3D bioprinting ranging from basic biological, material and technical advances to pre-clinical and clinical applications of 3D bioprinting.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


