Fenton(类)反应中的Fe(IV)和Cu(III)水溶液:化学和环境应用
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
通过芬顿(类)反应生成的高价金属(hvm)的化学性质在文献中得到了广泛的报道。与羟基自由基相比,羟基自由基是一种最有效但非选择性的氧化剂,对多种有机化合物表现出明显的反应性,hvm被认为是一种高活性的选择性氧化剂。由于其目标特异性行为,基于hvm的氧化工艺在水和废水处理中的应用引起了相当大的关注。本文主要综述了铁基铜和三价铜(即FeIVO2+和Cu(III)),它们是与环境最相关且被广泛研究的hvm。研究了这些物质通过芬顿(类)反应的形成机制,然后详细讨论了它们的化学性质,包括结构特征、氧化还原电位和水解行为。然后讨论了hvm对各种有机分子的独特反应性与底物结构和相应的氧化机制的关系。本文还综述了检测fevo2 +和Cu(III)的分析方法,包括化学探针技术、基于清除剂的抑制试验、电子顺磁共振和其他补充方法。本文介绍了不同fevo2 +-和Cu(III)基均相和非均相系统的案例研究,以说明它们在有机污染物降解和微生物灭活中的实际应用,以及水基质成分对处理效果的影响。最后,展望了将基于hvm的氧化作为一种可持续和有效的水处理策略所需要的重点研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
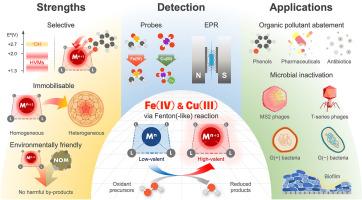
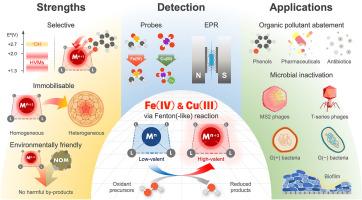
Aqueous Fe(IV) and Cu(III) species from Fenton (-like) reactions: Chemistry and environmental applications
The chemistry of high-valent metal species (HVMs) generated via Fenton (-like) reactions has been extensively reported in the literature. HVMs are recognised as highly reactive yet selective oxidants, in contrast to hydroxyl radicals—one of the most potent but nonselective oxidants—exhibiting distinct reactivity towards a broad range of organic compounds. Owing to their target-specific behaviour, HVM-based oxidation processes have garnered considerable attention for applications in water and wastewater treatment. This review focuses on ferryl and trivalent copper species (i.e. FeIVO2+ and Cu(III)), which are among the most environmentally relevant and widely studied HVMs. The formation mechanisms of these species via Fenton (-like) reactions are examined, followed by a detailed discussion of their chemical properties, including structural features, redox potentials, and hydrolytic behaviours. The unique reactivity of HVMs towards diverse organic molecules is then discussed in relation to substrate structures and corresponding oxidation mechanisms. Analytical methods for detecting FeIVO2+ and Cu(III) are also reviewed, including chemical probe techniques, scavenger-based inhibition assays, electron paramagnetic resonance, and other complementary approaches. Case studies of diverse FeIVO2+- and Cu(III)-based homogeneous and heterogeneous systems are presented to illustrate their practical applications in organic pollutant degradation and microbial inactivation, as well as the influence of water matrix components on treatment efficacy. The review concludes with a perspective on key research directions needed to advance HVM-based oxidation as a sustainable and effective strategy for water treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


