EHA-EMN多发性骨髓瘤的诊断、治疗和随访循证指南
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
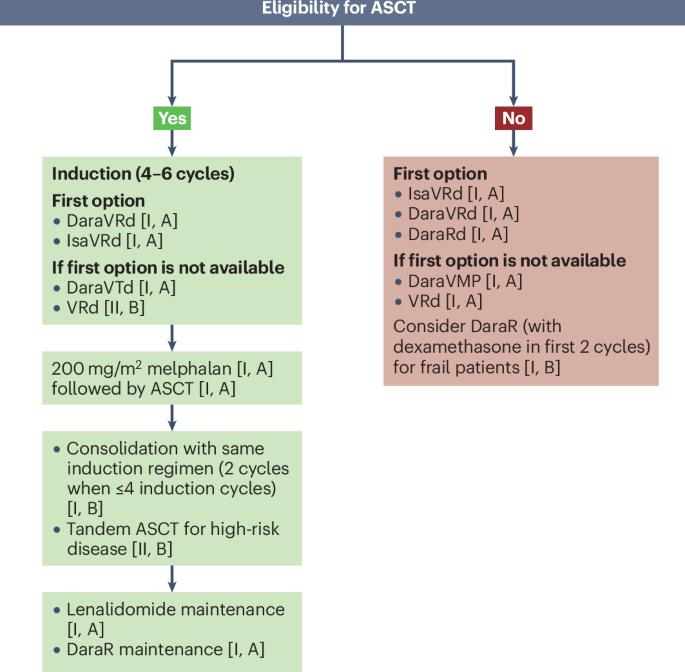
自欧洲血液学协会(EHA)与欧洲肿瘤医学学会合作制定的阴燃型多发性骨髓瘤(SMM)和多发性骨髓瘤(MM)患者治疗临床实践指南于2021年出版以来,一种新的国际分期系统(R2-ISS)已经开发出来,一些预后因素正在进入临床实践(如最小残留病、在撰写本文时,EMA和/或FDA已经批准了14种用于治疗MM患者的新方案。来自EHA和欧洲骨髓瘤网络的多学科专家小组,主要位于欧洲的不同机构,更新了以前的指南,并根据上述新数据制定了纳入证据水平和推荐等级的日常临床实践算法。在这些循证指南中,我们为新诊断的MM患者和复发和/或难治性MM患者提供了关键的治疗建议,包括现有药物和当代免疫疗法的使用指南。管理SMM患者的新方法侧重于那些可能需要早期干预的患者。最后,我们提供了骨髓瘤相关并发症和不良事件的建议,如骨病、肾脏损害和感染,以及与T细胞动员疗法相关的并发症和不良事件,如细胞因子释放综合征和免疫效应细胞相关神经毒性综合征。本文章由计算机程序翻译,如有差异,请以英文原文为准。

EHA–EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma
Since the publication in 2021 of the European Hematology Association (EHA) Clinical Practice Guidelines for the treatment of patients with smouldering multiple myeloma (SMM) and multiple myeloma (MM), developed in collaboration with the European Society for Medical Oncology, a novel international staging system (R2-ISS) has been developed, several prognostic factors are entering clinical practice (such as minimal residual disease, circulating plasma cells and monoclonal protein assessed by mass spectrometry) and, at the time of writing, 14 novel regimens have been approved by the EMA and/or the FDA for the treatment of patients with MM. A multidisciplinary group of experts from the EHA and European Myeloma Network, based in various institutions mostly located in Europe, have updated the previous guidelines and produced algorithms for everyday clinical practice that incorporate levels of evidence and grades of recommendation based on the aforementioned new data. In these Evidence-Based Guidelines, we provide key treatment recommendations for both patients with newly diagnosed MM and those with relapsed and/or refractory MM, including guidance for the use of established drugs as well as contemporary immunotherapies. Novel approaches for the management of patients with SMM focus on those who might require early intervention. Finally, we provide recommendations for myeloma-related complications and adverse events, such as bone disease, renal impairment and infections, as well as for those associated with T cell-mobilizing therapies, such as cytokine-release syndrome and immune effector cell-associated neurotoxicity syndrome. In these Evidence-Based Guidelines, a multidisciplinary panel of experts from the European Hematology Association and the European Myeloma Network provide key treatment recommendations for patients with smouldering multiple myeloma, and newly diagnosed or relapsed and/or refractory multiple myeloma, addressing the use of established drugs and novel immunotherapies as well as the management of disease-specific and treatment-related complications and adverse events.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


