环己酮好氧合成邻苯二胺的研究
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
提出了一种碘铜共催化环己酮直接转化为邻苯二胺的新方法。这种转变是值得注意的,因为它与各种胺的相容性,因为它在温和的条件下利用空气作为绿色氧化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
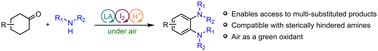
Aerobic oxidative synthesis of o-phenylenediamines from cyclohexanones†
A novel iodine and copper co-catalyzed method for the direct conversion of cyclohexanones into substituted o-phenylenediamines has been developed. This transformation is notable for its compatibility with a variety of amines and because it utilizes air as a green oxidant under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


