苯甲醛裂解酶高效合成二羟基丙酮及一锅生物合成2-氨基-1,3-丙二醇†的结构导向工程
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
2-氨基-1,3-丙二醇(丝氨酸醇)普遍存在于生物活性化合物和药物制剂中。二羟丙酮(DHA)作为丝氨酸醇的关键前体,在丝氨酸醇的生物合成中起着至关重要的作用。甲酰基甲酸酶(FLS)催化C1复合甲醛(HCHO)的炭化反应被认为是制备DHA的一种极具价值的方法。迄今为止,仅从荧光假单胞菌生物变种I中工程发现了苯甲醛裂解酶(BAL)和从恶臭假单胞菌中工程发现了苯甲酰甲酸脱羧酶(BFD),但其活性和化学选择性较低,阻碍了其工业应用。在本研究中,通过对来自于除草剂的sav - r1的BAL进行工程修饰,发现了一种具有高催化效率(14.60 s−1 M−1)和良好化学选择性(>99%)的合成DHA的FLS。同时,分子动力学研究表明,减少结合袋的体积可以显著稳定酶中的辅因子,从而提高FLS的活性。此外,通过在野生型BALs的活性口袋中引入6个关键突变,成功获得了一系列新的fls。此外,设计并实现了fls催化HCHO碳化和转氨酶转氨化的一锅两步酶促工艺,为从HCHO生产丝氨酸醇(分离率91%)提供了一种新的高效方法,从而以廉价和环保的方式促进了C1化合物的价化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
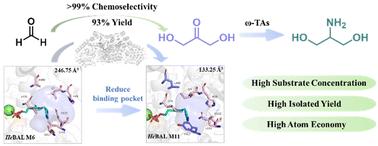
Structure-guided engineering of benzaldehyde lyase for efficient synthesis of dihydroxyacetone and one-pot biosynthesis of 2-amino-1,3-propanediol†
2-Amino-1,3-propanediol (serinol) is ubiquitous in bioactive compounds and pharmaceutical agents. As a key precursor, dihydroxyacetone (DHA) plays an essential role in the biosynthesis of serinol. The carboligation of C1 compound formaldehyde (HCHO) catalyzed by formolase (FLS) is considered as a highly valuable approach to obtain DHA. Until now, only two FLSs have been discovered by engineering the benzaldehyde lyase (BAL) from Pseudomonas fluorescens biovar I and the benzoylformate decarboxylase (BFD) from Pseudomonas putida, but their low activity and chemoselectivity hinder their industrial application. In this study, a novel FLS with high catalytic efficiency (14.60 s−1 M−1) and excellent chemoselectivity (>99%) for the synthesis of DHA was discovered by engineering a BAL from Herbiconiux sp. SALV-R1. Meanwhile, molecular dynamics studies demonstrated that reducing the volume of the binding pocket significantly stabilized the cofactor in the enzyme, which in turn increased the activity of FLS. In addition, a series of de novo FLSs were successfully obtained by introducing six key mutations within the active pocket of wild-type BALs. Furthermore, a one-pot, two-step enzymatic process involving FLS-catalyzed carboligation of HCHO and subsequent transamination with transaminases was designed and implemented, providing a new and efficient method to produce serinol (91% isolated yield) from HCHO and thus contributing to the valorization of C1 compounds in a cheap and environment-friendly way.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


