电化学氧促进IrO2催化剂实现甲烷室温部分氧化制甲醇的高选择性
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
甲烷的电化学转化为增值化学品为利用丰富的甲烷资源提供了一个可持续的解决方案,但实现部分氧化的高选择性仍然是一个挑战。在这里,我们证明了使用CO32 -作为氧源的IrO2催化剂可以在室温下实现高效和选择性的电化学甲烷到甲醇的转化。CO32−在IrO2(110)表面的吸附和解离产生丰富的活性氧,促进甲烷通过表面结合的甲氧基中间体活化,从而大大提高甲醇的选择性。甲醇生产的最佳条件是在一个电位范围内,从竞争析氧反应的干扰是最小的,达到最大的甲醇生产速率约11.1 mmol gcat - 1 h - 1与可逆氢电极在1.50连续操作。过程建模表明,与传统的甲醇生产方法相比,碳排放量减少了约50%,强调了这种电化学甲烷氧化方法的可持续性和实用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
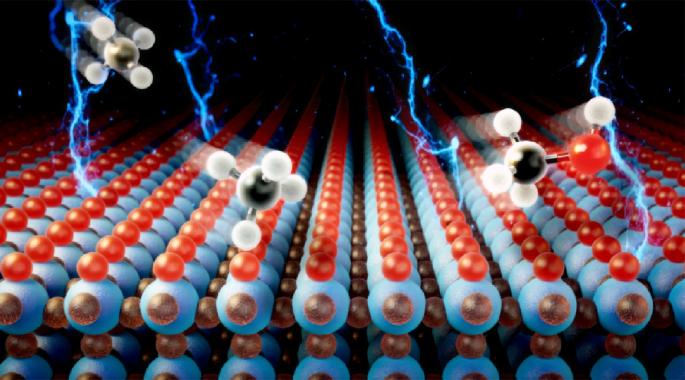

High-selectivity room-temperature partial oxidation of methane to methanol enabled by electrochemical oxygen promotion on IrO2 catalysts
The electrochemical conversion of methane into value-added chemicals offers a sustainable solution for utilizing abundant methane resources, yet achieving high selectivity for partial oxidation remains challenging. Here we demonstrate that employing an IrO2 catalyst with CO32− as an oxygen source enables efficient and selective electrochemical methane-to-methanol conversion at room temperature. Adsorption and dissociation of CO32− on IrO2(110) surfaces generates abundant active oxygen species, facilitating methane activation through surface-bound methoxy intermediates and thereby substantially enhancing methanol selectivity. Optimal conditions for methanol production are achieved within a potential range where interference from the competing oxygen evolution reaction is minimized, reaching a maximum methanol production rate of approximately 11.1 mmol gcat−1 h−1 at 1.50 versus the reversible hydrogen electrode under continuous operation. Process modelling indicates an approximately 50% reduction in carbon emissions compared to conventional methanol production methods, emphasizing the sustainability and practical potential of this electrochemical methane oxidation approach. The electrochemical oxidation of methane is a promising process but controlling its selectivity for a partial oxidation product such as methanol is very challenging. Now a strategy to convert methane into methanol with high selectivity is demonstrated, using an IrO2 catalyst and CO32− in the electrolyte as the oxygen source.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


