短链氯化石蜡在环境相关剂量下通过巨噬细胞介导的炎症破坏葡萄糖稳态和胰岛素信号
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
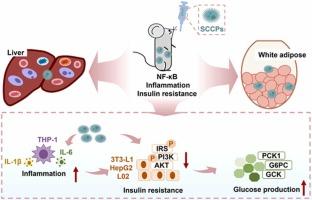
短链氯化石蜡(SCCPs)普遍存在于环境基质中,已在多种人体生物标本中检测到。虽然高剂量的SCCP暴露可诱发肝、肾和甲状腺毒性,但与环境相关剂量的SCCP的健康风险仍不清楚。本研究系统地研究了环境相关剂量的SCCPs对葡萄糖稳态和胰岛素信号传导的影响,并探讨了其潜在机制。研究发现,亚急性SCCP暴露(1-50 mg/kg bw)会降低胰岛素和葡萄糖耐量,增加血清胰岛素水平,并损害小鼠肝脏和脂肪组织中的胰岛素信号活性。胰岛素抵抗的稳态模型评估(HOMA-IR)分别从对照组的2.53增加到1、5、10和50 mg/kg bw SCCP组的2.98、3.22、3.86和4.85。SCCP暴露引发了这些组织的炎症,这可以通过巨噬细胞浸润、核因子κB (NF-κB)激活以及Il6、Il1b和Tnfa mRNA表达上调来证明。体外肝细胞-巨噬细胞共培养系统实验表明,SCCPs (100 μg/L)诱导的肝细胞胰岛素抵抗是由活化的巨噬细胞介导的。值得注意的是,药理NF-κB抑制剂(JSH-23)和细胞因子中和(IL6和IL1β)可显著改善sccp诱导的肝细胞胰岛素抵抗。这些发现揭示了胰岛素信号是环境相关剂量SCCP暴露的一个以前未被认识的敏感靶点,并确定巨噬细胞介导的炎症是一个关键机制。本研究仅研究了SCCP暴露对雄性小鼠胰岛素信号的亚急性影响。慢性SCCP暴露对胰岛素信号的影响和潜在的性别差异值得进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Short-chain chlorinated paraffins at environmentally relevant doses disrupt glucose homeostasis and insulin signaling by macrophage-mediated inflammation
Short-chain chlorinated paraffins (SCCPs) are ubiquitously distributed in environmental matrices and have been detected in diverse human biospecimens. While high-dose SCCP exposure can induce liver, kidney, and thyroid toxicity, the health risks of SCCPs at environmentally relevant doses remain poorly characterized. This study systematically investigated the impacts of SCCPs at environmentally relevant doses on glucose homeostasis and insulin signaling and explored the underlying mechanisms. It was found that subacute SCCP exposure (1-50 mg/kg bw) compromised insulin and glucose tolerance, increased serum insulin levels, and impaired insulin signaling activity in mouse liver and adipose tissues. The homeostasis model assessment of insulin resistance (HOMA-IR) was increased from 2.53 in control group to 2.98, 3.22, 3.86, and 4.85 in 1, 5, 10, and 50 mg/kg bw SCCP groups, respectively. SCCP exposure triggered inflammation in these tissues, evidenced by macrophage infiltration, nuclear factor kappa B (NF-κB) activation, and upregulated Il6, Il1b, and Tnfa mRNA expression. In vitro experiments employing a hepatocyte-macrophage co-culture system revealed that SCCPs (100 μg/L) -induced insulin resistance in hepatocytes was mediated by activated macrophages. Notably, pharmacological NF-κB inhibitor (JSH-23) and cytokine neutralization (IL6 and IL1β) significantly ameliorated SCCP-induced insulin resistance in hepatocytes. These findings reveal insulin signaling as a previously unrecognized sensitive target of SCCP exposure at environmentally relevant doses and identify macrophage-mediated inflammation as a pivotal mechanism. This study only addressed the subacute effects of SCCP exposure on insulin signaling in male mice. The effects of chronic SCCP exposure on insulin signaling and potential gender differences warrant further investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


