高温诱导的FKF1积累通过GI的分散和SVP的降解促进开花。
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
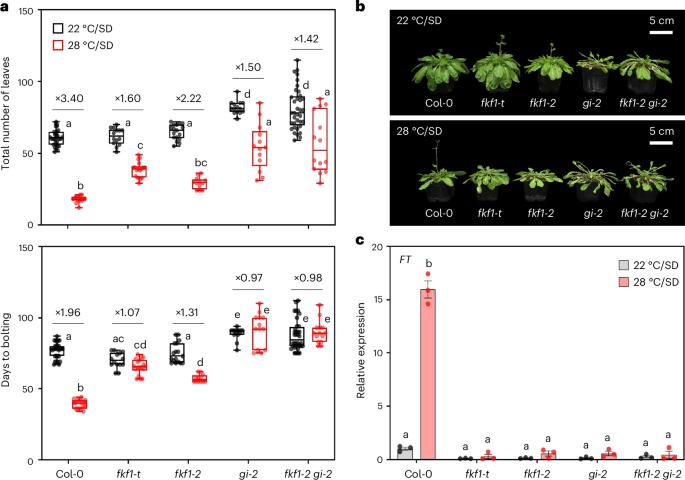
花的转变受光周期和环境温度的影响,它们通过一个分子机制来调节发育,这一机制仍有待阐明。在这里,我们证明了F-box蛋白黄素结合,KELCH REPEAT, F-box 1 (FKF1)及其相互作用伙伴GIGANTEA (GI),光周期开花的中心调控因子,靶向短营养期(SVP)进行26s蛋白酶体依赖性降解,以调节温度响应性发育过渡到开花。在低温下,GI被封存在液态核凝析物中。相反,FKF1在高温下积累,并从凝析油中释放GI,形成核分散的FKF1-GI复合物,导致SVP在短日条件下降解。fkf1-t、gi-2和fkf1-2 - gi-2突变体的温度敏感性显著降低。我们提出FKF1-GI复合物通过可逆的液-液相分离介导花抑制因子的蛋白水解,从而加速高温下的花转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。

High-temperature-induced FKF1 accumulation promotes flowering through the dispersion of GI and degradation of SVP
Floral transition is influenced by photoperiod and ambient temperature, which are integrated to modulate development via a molecular mechanism that remains to be elucidated. Here we demonstrate that the F-box protein FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1) and its interacting partner GIGANTEA (GI), central regulators of photoperiodic flowering, target SHORT VEGETATIVE PHASE (SVP) for 26S-proteasome-dependent degradation to regulate the temperature-responsive developmental transition to flowering. At low temperatures, GI is sequestered in liquid-like nuclear condensates. By contrast, FKF1 accumulates at high temperatures and releases GI from condensates to form a nuclear-dispersed FKF1–GI complex, leading to SVP degradation under short-day conditions. Temperature sensitivity is significantly reduced in fkf1-t, gi-2 and fkf1-2 gi-2 mutants. We propose that the FKF1–GI complex mediates the proteolysis of a floral repressor via reversible liquid–liquid phase separation to accelerate floral transition at high temperatures. GI forms an inactive nuclear condensate that is dispersed at high temperatures by FKF1 binding to GI’s intrinsically disordered region. The resulting FKF1–GI complex promotes SVP degradation, accelerating flowering at high ambient temperatures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


