植物病原菌祖先抗菌分泌系统的功能替代。
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
两种不同的分子机制,T4SS和T6SS,已经在黄单胞菌目中被发现,通过分泌毒性效应蛋白来参与与其他细菌物种的竞争。然而,导致黄单胞菌进化出两种功能相似的分泌系统的生态和进化基础尚不清楚。在这里,我们发现透光黄单胞菌(Xt)谱系已经从x - t4ss介导的细菌杀伤策略转变为t6ss -i4介导的细菌杀伤策略。T6SS-i4仅存在于缺乏X-T4SS的Xt菌株中,反之亦然,导致两种纳米武器沿Xt系统发育呈斑块状分布。利用遗传和荧光方法,我们证明了X-T4SS和T6SS-i4对Xt的细菌间竞争至关重要,而不是Xt T6SS-i3。结合比较遗传和系统发育分析进一步发现,X-T4SS基因簇存在退化和多个丢失事件,而T6SS-i4基因簇通过独立的增益事件插入。总的来说,本研究支持了X-T4SS的祖先状态,并为促进X-T4SS在生态位内生存的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
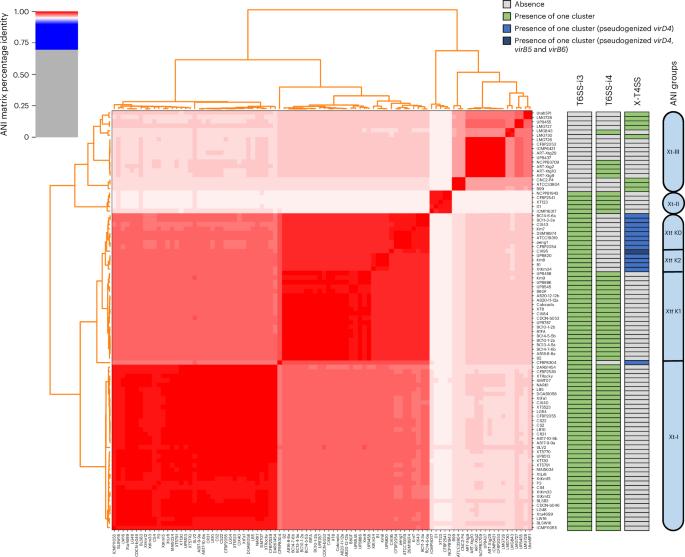

Functional replacement of ancestral antibacterial secretion system in a bacterial plant pathogen
Two distinct molecular machineries, T4SS and T6SS, have been found within the order Xanthomonadales to be involved in outcompeting other bacterial species through the secretion of toxic effector proteins. However, the ecological and evolutionary basis leading xanthomonads to evolve two secretion systems with such similar functions remain unclear. Here we show that Xanthomonas translucens (Xt) lineages have switched from an X-T4SS-mediated to T6SS-i4-mediated bacterial killing strategy. T6SS-i4 was only found in Xt strains lacking X-T4SS and vice versa, resulting in a patchy distribution of the two nanoweapons along the Xt phylogeny. Using genetic and fluorescence-based methods, we demonstrated that X-T4SS and T6SS-i4 are crucial for interbacterial competition in Xt, but not Xt T6SS-i3. Combined comparative genetic and phylogenetic analyses further revealed that the X-T4SS gene clusters have been subject to degradation and had several loss events, while T6SS-i4 was inserted through independent gain events. Overall, this research supports the ancestral state of X-T4SS and provides new insights into the mechanisms promoting Xt survival within their ecological niches. The bacterial plant pathogen genus Xanthomonas uses two distinct secretion systems for antibacterial competition. Here the authors show that some Xanthomonas lineages have switched from the ancestral X-T4SS state to T6SS-i4 despite the functional similarity of the systems, with X-T4SS gene clusters subject to degradation and loss and T6SS-i4-encoding sequences inserted through independent gains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


