邻乙基苯甲醛和苯胺温和高效地构建含氮杂环。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用邻乙基苯甲醛和苯胺合成了一种温和的、无过渡金属的异吲哚-1-亚胺和异喹啉盐。HFIP被用作有效的促进剂,促进亲核加成和环化,以高产率获得多种异吲哚-1-亚胺。或者,通过添加碘化铵得到异喹啉盐。这两种反应都表现出广泛的官能团耐受性,并成功地应用于克尺度和进一步转化,为药物开发和有机合成提供了实用的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
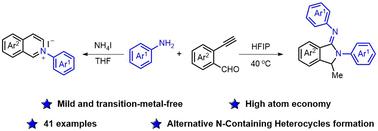
Mild and efficient construction of nitrogen-containing heterocycles from ortho-ethynylbenzaldehydes and anilines†
A mild and transition-metal-free strategy for the synthesis of isoindolin-1-imines and isoquinolinium salts has been developed using ortho-ethynylbenzaldehydes and anilines. HFIP was employed as an effective promoter, facilitating nucleophilic addition and annulation to afford diverse isoindolin-1-imines in good yields. Alternatively, isoquinolinium salts were obtained by addition of ammonium iodide. Both reactions exhibited broad functional group tolerance and were successfully applied on gram scales as well as for further transformation, providing a practical strategy in pharmaceutical development and organic synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


