碱性介质中氧还原反应的无负载和碳负载银催化剂
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
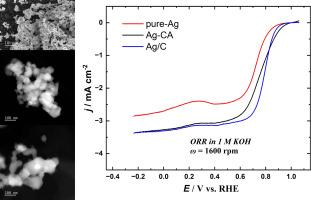
仅使用还原剂(纯银),湿法化学合成快速简便制备银催化剂还原剂和柠檬酸作为封盖剂(Ag-CA);碳载体(KetjenBlack 600J)、封盖剂和还原剂(Ag/C)进行了论证。采用高角环形暗场扫描透射电子显微镜(HAADF-STEM)、能量色散x射线能谱(EDS)、扫描电子显微镜(SEM)、x射线衍射(XRD)和x射线光电子能谱(XPS)对银基电催化剂进行了表征。采用旋转(环)盘电极法评价了银催化剂在1 M KOH中O2还原反应(ORR)的电催化活性。SEM和HAADF-STEM结果表明,无负载的纯Ag和Ag- ca催化剂主要由大团聚体组成,Ag/C催化剂的团聚体最小,并含有一些亚3 nm的Ag纳米颗粒。XPS结果表明,所有催化剂中的Ag均以金属形式存在(Ag0)。Ag- ca催化剂虽然由大团块组成,但其ORR电催化活性与Ag/C相似。Ag-CA(无负载)的过氧化氢产率最低。这些结果对于开发快速、简单且易于扩展的银基燃料电池催化剂具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unsupported and carbon-supported silver catalysts for oxygen reduction reaction in alkaline media
Quick and easy Ag catalysts preparation via wet chemical synthesis method using only reducing agent (pure-Ag); reducing agent and citric acid as the capping agent (Ag-CA); and carbon support (KetjenBlack 600J), capping agent, and the reducing agent (Ag/C) is demonstrated. The Ag-based electrocatalysts are characterized by high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) with energy-dispersive X-ray spectroscopy (EDS), scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity of Ag catalysts for O2 reduction reaction (ORR) in 1 M KOH is evaluated using the rotating (ring)-disc electrode method. SEM and HAADF-STEM results show that the unsupported pure-Ag and Ag-CA catalysts consist mainly of big agglomerates, and Ag/C has the smallest agglomerates and some sub-3 nm Ag nanoparticles. The XPS results reveal that Ag in all the catalysts is in the metallic form (Ag0). Despite consisting of big agglomerates, the Ag-CA catalyst exhibits similar ORR electrocatalytic activity to that of Ag/C. Ag-CA (unsupported) shows the lowest hydrogen peroxide yield. These results are of great importance for the development of Ag-based catalysts that can be prepared in a fast, simple and easily up scalable fashion, for anion exchange membrane fuel cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


