snRNA输出复合物协同组装的结构基础
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
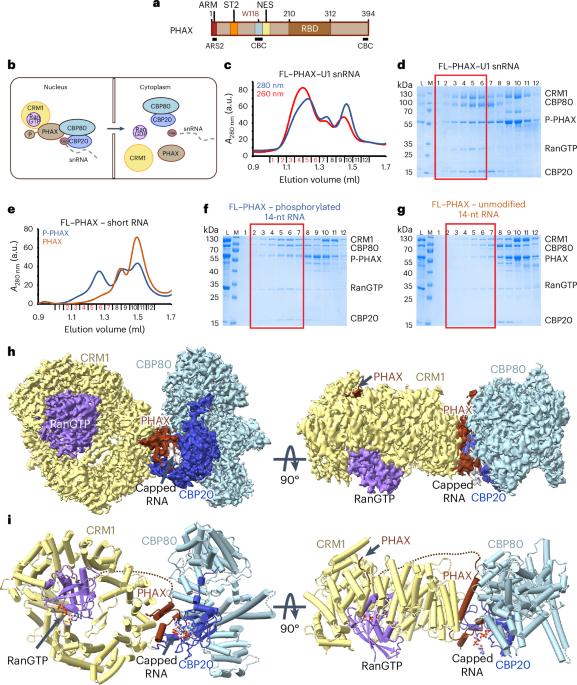
核帽结合复合体(CBC)及其伙伴亚砷酸盐抗性蛋白2 (ARS2)通过与RNA效应物的互斥相互作用调节RNA聚合酶II转录本的命运。其中一种效应物是PHAX,它介导富铀小核rna (snrna)的核输出。在这里,我们展示了人类snRNA输出复合体的低温电镜结构,该复合体包括磷酸化的PHAX、CBC、CRM1-RanGTP和盖帽RNA。PHAX的中心区域将CBC与输出因子CRM1-RanGTP连接起来,同时也加强了帽二核苷酸的结合。此外,PHAX与CRM1的远端区域相互作用,促进了PHAX的必需磷酸化区域与RanGTP的突出基面接触。CBC与snRNA输出复合体的结合与其与其他RNA效应物(如ALYREF或NCBP3)的结合不相容。我们证明,snRNA输出复合体的形成需要其所有组分的协同结合,这反过来取代了CBC中的ARS2,并将该复合体用于输出。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Structural basis for the synergistic assembly of the snRNA export complex
The nuclear cap-binding complex (CBC) and its partner Arsenite-Resistance Protein 2 (ARS2) regulate the fate of RNA polymerase II transcripts via mutually exclusive interactions with RNA effectors. One such effector is PHAX, which mediates the nuclear export of U-rich small nuclear RNAs (snRNAs). Here we present the cryo-electron microscopy structure of the human snRNA export complex comprising phosphorylated PHAX, CBC, CRM1–RanGTP and capped RNA. The central region of PHAX bridges CBC to the export factor CRM1–RanGTP, while also reinforcing cap dinucleotide binding. Additionally, PHAX interacts with a distant region of CRM1, facilitating contacts of the essential phosphorylated region of PHAX with the prominent basic surface of RanGTP. CBC engagement within the snRNA export complex is incompatible with its binding to other RNA effectors such as ALYREF or NCBP3. We demonstrate that snRNA export complex formation requires synergistic binding of all its components, which in turn displaces ARS2 from CBC and commits the complex for export. Dubiez and colleagues present a cryo-EM structure of the complex responsible for nuclear export of pre-small nuclear RNAs, comprising CBC–PHAX–CRM1–RanGTP. The structure provides insights into the complex architecture, assembly and target RNA recognition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


