烯基肟的电化学三氟甲基化,通过自由基环化合成异恶唑啉和环硝酮。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种高效、环保的方法,可在不添加任何氧化剂的情况下获得含氟异恶唑啉和环硝基酮衍生物。在该电化学体系中,三氟甲烷磺酸钠(CF3SO2Na)作为一种实用且廉价的三氟甲基源,可以与各种β,γ-或γ,δ-烯基肟底物进行电化学诱导自由基加成和氧化环化。该工艺在温和的条件下顺利进行,并表现出广泛的底物相容性,可耐受供电子和吸电子取代基。机理研究和循环伏安实验表明,三氟甲基自由基在电化学条件下优先引发。所需要的产品以中等到良好的收率得到。此外,通过克级反应证明了该方法的合成效用,该反应使目标化合物的效率略有降低。这项工作为构建cf3官能化杂环提供了一个绿色和可扩展的平台,为可持续自由基氟化策略提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
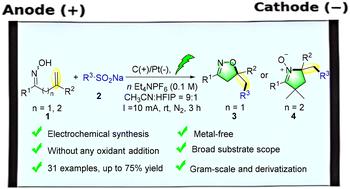
Electrochemical trifluoromethylation of alkenyl oximes for the synthesis of isoxazolines and cyclic nitrones via radical annulation†
A highly efficient and environmentally friendly approach to access fluorine-containing isoxazoline and cyclic nitrone derivatives without any oxidant addition is reported. In this electrochemical system, sodium trifluoromethanesulfonate (CF3SO2Na) serves as a practical and inexpensive trifluoromethyl source, enabling electrochemically induced radical addition and oxidative cyclization with a variety of β,γ- or γ,δ-alkenyl oxime substrates. This protocol proceeds smoothly under mild conditions and exhibits broad substrate compatibility, tolerating both electron-donating and electron-withdrawing substituents. Mechanistic studies and cyclic voltammetry experiments indicate that the trifluoromethyl radical is preferentially initiated under electrochemical conditions. The desired products are obtained in moderate to good yields. Furthermore, the synthetic utility of this methodology was demonstrated by a gram-scale reaction, which furnished the target compound with slightly reduced efficiency. This work provides a green and scalable platform for the construction of CF3-functionalized heterocycles, offering valuable insights into sustainable radical fluorination strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


