乙醇酸淀粉钠交联强度的定量测定及其对片剂崩解溶出的影响
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-27
DOI:10.1016/j.ejpb.2025.114803
引用次数: 0
摘要
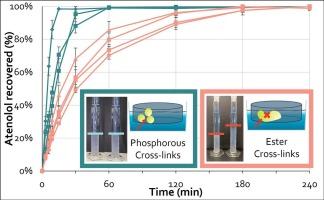
开发坚固耐用的口服固体剂型需要了解可能影响剂型性能和稳定性的所有变异来源,符合质量设计(QbD)。这需要对原材料特性、工艺参数和最终产品质量之间的关系有深刻的理解。本文提出了一种量化SSG等级中交联强度(SXL)的方法,通过比较暴露于碱性条件前后胶凝后降水的沉积物体积。结果表明,具有磷交联的SSG牌号比酯交联更耐水解。所开发的方法可以帮助配方师在配方中使用前评估SSG的SXL。对7个已上市的SSG品级的SXL进行了量化,结果表明,具有磷交联(SXL≈1)和酯交联(SXL 0.4 ~ 0.7)的SSG品级之间存在明显差异。用阿替洛尔湿制颗粒剂对这些SSG等级的片剂崩解和原料药溶出度进行了评价。结果表明,所有含磷交联的SSG品级在25 min内都实现了80%的阿替洛尔溶解,而所有含酯交联的品级在45 min或更长时间后才达到这一阈值。这表明,交联的类型是导致溶解成功或失败的关键属性。在SXL和80%阿替洛尔溶解的时间之间观察到极好的相关性。这项研究的结果表明,了解交联的类型和SSG的SXL可以支持配方师根据设计质量方法开发出良好、可靠的配方。所提供的案例研究表明,配方制造商可以通过使用含磷交联的SSG级,使其配方在片剂生产之前、期间或之后对压力暴露更强,从而限制了产品失效的风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quantifying cross-linking strength in sodium starch glycolate and its impact on tablet disintegration and dissolution
The development of a robust oral solid dosage form requires knowledge of all the sources of variation that could impact the dosage form’s performance and stability, in line with Quality-by-Design (QbD). This requires a deep understanding of the relationships between raw material properties, the process parameters and the final product quality. This paper presents a method to quantify the strength of cross-links (SXL) in SSG grades by comparing the sediment volume of precipitation after gelling before and after exposure to alkaline conditions. It is demonstrated that SSG grades with phosphorous cross-links are more resistant to hydrolysis than ester cross-links. The developed method can help formulators to assess the SXL of SSG before use in a formulation. The SXL was quantified for seven marketed SSG grades, showing a clear difference between SSG grades with phosphorous cross-links (SXL ≈ 1) and ester cross-links (SXL 0.4 – 0.7). The tablet disintegration and API dissolution of these SSG grades were evaluated for a wet granulation formulation with atenolol. Results show that all SSG grades with phosphorous cross-links realized 80 % atenolol dissolution within 25 min, whereas all grades with ester cross-links reached this threshold only after 45 min or more. This shows that the type of cross-link is a critical attribute that can result in dissolution success or failure. An excellent correlation was observed between the SXL and the time at which 80 % of the atenolol was dissolved. The findings of this study show that understanding the type of cross-link and the SXL of SSG can support formulators with the development of good, robust formulations, in line with a quality-by-design approach. The provided case study shows that formulators can make their formulations more robust to stress exposure before, during or after tablet manufacturing by using an SSG grade with phosphorous cross-links, thereby limiting the risk of product failure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


