蒙脱土在单、二元体系中对农药吸附潜力的研究
IF 5.8
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
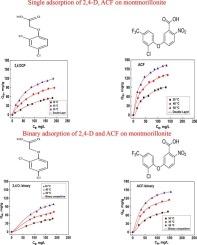
在不同温度(30、40、50℃)下,获得了2,4-二氯苯氧乙酸(2,4- d)和氟氯芬(ACF)在蒙脱土上吸附的单化合物和二元实验数据,以解释和理解这些水污染物之间的竞争相互作用。实验结果表明,ACF在单化合物体系中的最大吸附量分别约为90、130和161 mg/g。在二元体系中,这些容量分别下降到73,114和142 mg/g。对于2,4- d,单一化合物的吸附量分别为55、89 ~ 119 mg/g,而二元化合物的吸附量分别为44、71和98 mg/g。吸附性能的降低与所研究分子之间的竞争效应有关。为了进一步阐明其在单化合物和二元体系中的吸附机理,在模型分析的基础上进行了理论研究。采用单化合物的双层吸附模型和二元体系的竞争吸附模型对可能的机理进行了深入研究。结果表明,ACF通过聚集过程吸附,在较低温度(30°C)下形成三聚体,而在较高温度(50°C)下这种聚集减少,导致单体形成。相反,2,4- d不经过聚集过程就被去除。二元模型显示,溶液中第二种化合物的存在产生拮抗作用,导致吸附能力降低。该理论方法表明,ACF和2,4- d主要通过物理相互作用被吸附,吸热过程在它们的去除中起关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of montmorillonite clay for its potential application for pesticides adsorption in single and binary systems
Single-compounds and binary experimental data on the adsorption of 2,4-Dichlorophenoxyacetic acid (2,4-D) and Acifluorfen (ACF) onto montmorillonite are obtained at different temperatures (30, 40, 50 °C) to explain and understand the competitive interactions between these water contaminants. The experimental results indicate that the maximum adsorption capacities of ACF in the single-compound system are approximately 90, 130, and 161 mg/g, respectively. In the binary system, these capacities decrease to 73, 114, and 142 mg/g, respectively. For 2,4-D, single-compound adsorption capacities varied from 55, 89 to 119 mg/g, while in binary system, they decreased to 44, 71 and 98 mg/g, respectively. The reduction in adsorption performance is related to the competitive effect between the investigated molecules. To further elucidate the adsorption mechanisms in single-compound and binary systems, a theoretical study based on modeling analysis is conducted. A double-layer adsorption model for single-compound and a competitive adsorption model for binary systems are employed to deeper investigate the possible mechanisms involved.
The results indicate that ACF is adsorbed via an aggregation process, forming trimers at lower temperatures (30 °C), whereas this aggregation is reduced at higher temperatures (50 °C), leading to monomer formation. In contrast, 2,4-D is removed without undergoing an aggregation process. Binary modeling revealed that the presence of a second compound in solution creates an antagonistic effect, leading to reduced adsorption capacities. This theoretical approach demonstrates that ACF and 2,4-D are adsorbed primarily through physical interactions, with endothermic processes playing a key role in their removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


