通过光催化化学选择性n-甲基丙烯醛腙的三/二氟甲基化和环化获得1-芳基-吡唑啉-5- 1。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以易获得的三氟甲基三氟化硫铵(TT-CF3+OTf-)为三氟甲基源,制备了三氟甲基化吡唑啉-5- 1衍生物(E)- n′-芳基芳基- n-苯基甲基丙烯酰肼的光催化三氟甲基化和5-内三环化反应。该方案具有广泛的底物范围和良好的化学选择性。在药物分子的后期修饰方面的应用进一步突出了当前策略的实用性。此外,在类似条件下,用PhI(O2CCHF2)2实现了二氟甲基化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
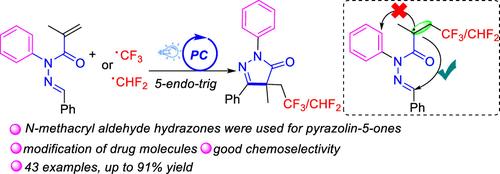
Access to 1-Aryl-pyrazolin-5-ones via Photocatalytic Chemoselective Tri/Difluoromethylation and Cyclization of N-Methacryloyl Aldehyde Hydrazones
A photocatalytic trifluoromethylation and 5-endo-trig cyclization of (E)-N′-arylidene-N-phenylmethacrylohydrazides was developed for the construction of trifluoromethylated pyrazolin-5-one derivatives using easily available trifluoromethyl thianthrenium triflate (TT-CF3+OTf–) as the trifluoromethyl source. This protocol exhibits a broad substrate scope and excellent chemoselectivity. The utility of the current strategy is further highlighted by its application in the late-stage modification of drug molecules. Furthermore, difluoromethylative cyclization was realized using PhI(O2CCHF2)2 under similar conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


