基于对羟基苯甲酸的新型三元深共晶溶剂木质素溶解机理的多尺度探索
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
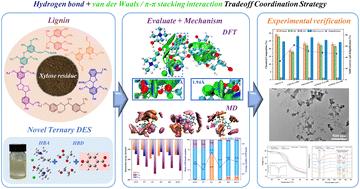
随着绿色化学概念的升级,木质纤维素处理中使用的溶剂对环境、经济和效率提出了更高的要求。由于缺乏对溶剂-生物质系统中分子水平机制的理解,仅关注增强系统内单一相互作用的研究受到了限制。本文采用氢键(h -键)协同作用的新策略合成了四种新型三元深共晶溶剂(DES)。利用范德华(vdW)相互作用和π -π堆叠增强DES的脱木质素能力。基于密度泛函数理论(DFT)和分子动力学(MD)模拟的量子化学计算结果表明,氯化胆碱(ChCl)和对羟基苯甲酸(PB)平均贡献了DES -木质素体系中84.26%和68.72%的静电能和vdW相互作用能,分别增加了21.60%和27.71%。这主要是由于ChCl上的N+增加了分子极性,sp3杂化N+周围的甲基排列增强了分散吸引力,PB和木质素的苯环结构之间形成π -π堆叠相互作用,从而增强了体系内的vdW相互作用。受苯环上邻位取代基的影响,6种木质素二聚体中β-1和β-O-4与DES的相互作用较强。ChCl-LA-PB处理木糖渣的实验表明,ChCl-LA-PB具有较强的脱木质素能力(计算得到的最强相互作用能达到- 92.37 kcal mol - 1)。与ChCl-LA相比,木质素的脱木质素率提高了1.86倍,达到61.91%。表征结果证实,酚醛羟基对PB的p -π偶联作用通过抑制H+的电离,避免了木质素的过度解聚和缩聚。从分子结构的起源出发设计DES,采用多作用协同的策略,为木质纤维素的高价值利用提供了新的思路和绿色途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multiscale exploration of the lignin dissolution mechanism based on novel ternary deep eutectic solvents incorporating p-hydroxybenzoic acid†
Heightened requirements in terms of the environment, economy, and efficiency have been imposed on solvents used in the treatment of lignocellulose by the upgrading of the concept of green chemistry. Limitations have been imposed on studies that only focus on enhancing a single interaction within a system due to the lack of understanding of the molecular-level mechanism in a solvent-biomass system. In this work, four novel ternary deep eutectic solvents (DES) were synthesized by adopting a new strategy in which the synergistic effect of hydrogen bonds (H-bonds), van der Waals (vdW) interactions and π–π stacking was used to enhance the delignification ability of DES. It was indicated by the results of quantum chemical calculations based on density functional theory (DFT) and molecular dynamics (MD) simulation that 84.26% and 68.72% of the electrostatic energy and vdW interaction energy in the DES–lignin system were contributed, on average, by choline chloride (ChCl) and p-hydroxybenzoic acid (PB), with increases of 21.60% and 27.71%, respectively. This improvement was attributed to the increased molecular polarity resulting from the N+ on ChCl, the enhanced dispersion attraction due to the arrangement of methyl groups around the sp3-hybridized N+, and the π–π stacking interaction formed between the benzene ring structure of PB and lignin, which resulted in the enhancement of vdW interactions within the system. Affected by the ortho-substituents on the benzene ring, among the six investigated lignin dimers, stronger interactions with DES were exhibited by β-1 and β-O-4. The delignification ability of ChCl–LA–PB (strongest calculated interaction energy, reaching −92.37 kcal mol−1) was demonstrated in the experiment of treating xylose residue. The delignification rate of lignin was increased by 1.86 times (up to 61.91%) compared with that of ChCl–LA. It was confirmed by the characterization result that the excessive depolymerization and condensation of lignin were avoided by the p–π conjugation effect of the phenolic hydroxyl group on PB through inhibiting the ionization of H+. Designing DES from the origin of the molecular structure and adopting a strategy of multiple interactions in synergy provided novel insights and green methods for the high-value utilization of lignocellulose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


