高频超声诱导苯胺衍生物在水中的无催化剂n -脱烷基反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这里,我们报道了由高频超声照射液体形成的空化气泡,诱导苯胺衍生物在水中的无催化剂n -脱烷基反应,从而为当前需要过渡金属和有害溶剂或氧化剂的技术开辟了一条替代途径。机理研究表明,苯胺衍生物的n -脱烷基反应发生在空化气泡-水界面,这是N-C键热裂解反应发生的区域。有利的是,由于其他组分在空化气泡-水界面上的强相互作用,苯胺衍生物的n -脱烷基反应也可以在其他组分存在的情况下特异性发生。与目前的裂解技术相比,这种将声波能量特定地转移到苯胺衍生物上的技术具有显著的优势,目前的裂解技术由于整个反应器的加热而导致非化学特定的裂解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
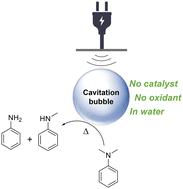
Catalyst-free N-dealkylation of aniline derivatives in water induced by high frequency ultrasound†
Here we report that cavitation bubbles, formed by the ultrasonic irradiation of a liquid at a high frequency, induce the catalyst-free N-dealkylation of aniline derivatives in water, thus opening an alternative pathway to current technologies which require transition metals and hazardous solvents or oxidants. Mechanism investigations revealed that the N-dealkylation of aniline derivatives takes place at the cavitation bubble–water interface, a region where N–C bond thermal cracking reactions occur. Advantageously, the N-dealkylation of aniline derivatives can also specifically occur in the presence of other components, thanks to their strong interaction at the cavitation bubble–water interface. This specific transfer of the acoustic energy to aniline derivatives represents a notable advantage over current cracking technologies, which lead to non-chemical specific cracking due to the heating of the whole reactor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


