细胞周期调节在出芽酵母中形成了复制起源
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
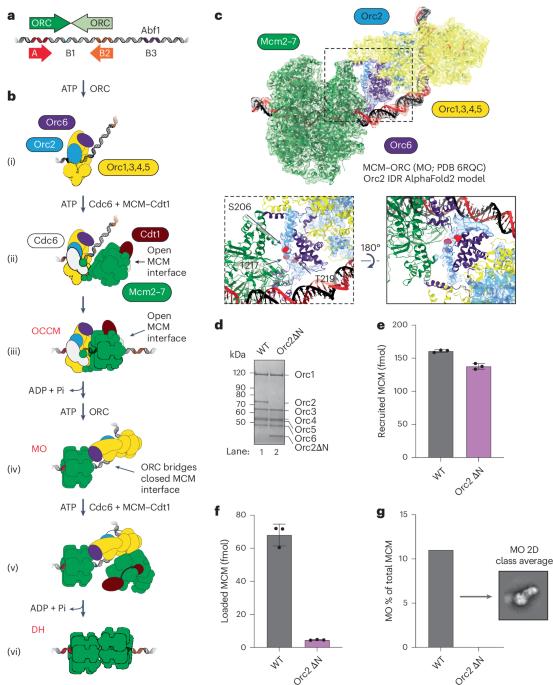
真核生物DNA复制开始于基因组位点,称为起源。在出芽酵母原点如ARS1, MCM复制解旋酶的双六聚体(DH)由原点识别复合体(ORC)、Cdc6和Cdt1通过从两个相反的ORC结合位点顺序加载六聚体组装而成。周期蛋白依赖性激酶(CDK)抑制DH组装,通过限制解旋酶加载到G1期来阻止再复制。在这里,我们发现Orc2亚基中的一个内在无序区(IDR)促进ORC与第一个加载的闭环MCM六聚体(MCM - ORC (MO)中间体)之间的相互作用。这种IDR的cdk依赖性磷酸化阻断了MO的形成和DH的组装。我们发现MO在低亲和力结合位点稳定ORC,这是第二次六聚体装载所需的。包含两个高亲和力ORC位点的起源可以通过独立加载单个六聚体来有效地组装DH而不需要MO。引人注目的是,这些起源在体外和体内都逃脱了CDK的抑制。我们的工作揭示了MCM加载的机制可塑性,这对理解CDK调控如何塑造酵母起源进化以及自然、强起源如何逃避细胞周期调控具有重要意义。我们还确定了加载途径的共同关键步骤,这对理解MCM如何在其他真核生物中加载具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Cell cycle regulation has shaped replication origins in budding yeast
Eukaryotic DNA replication initiates from genomic loci known as origins. At budding yeast origins like ARS1, a double hexamer (DH) of the MCM replicative helicase is assembled by origin recognition complex (ORC), Cdc6 and Cdt1 by sequential hexamer loading from two opposed ORC binding sites. Cyclin-dependent kinase (CDK) inhibits DH assembly, which prevents re-replication by restricting helicase loading to the G1 phase. Here, we show that an intrinsically disordered region (IDR) in the Orc2 subunit promotes interaction between ORC and the first loaded, closed-ring MCM hexamer (the MCM–ORC (MO) intermediate). CDK-dependent phosphorylation of this IDR blocks MO formation and DH assembly. We show that MO stabilizes ORC at lower-affinity binding sites required for second hexamer loading. Origins comprising two high-affinity ORC sites can assemble DH efficiently without MO by independently loading single hexamers. Strikingly, these origins escape CDK inhibition in vitro and in vivo. Our work reveals mechanistic plasticity in MCM loading with implications for understanding how CDK regulation has shaped yeast origin evolution and how natural, strong origins might escape cell cycle regulation. We also identify key steps common to loading pathways, with implications for understanding how MCM is loaded in other eukaryotes. Licensing of eukaryotic origins of replication with MCM double hexamers (DHs) can occur through distinct pathways. Here, Lim et al. show that in yeast, cell cycle-dependent regulation of DH formation by CDK and origin structure have co-evolved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


