CD4+ T细胞允许Kupffer细胞逆转由肝细胞启动诱导的CD8+ T细胞功能障碍
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
慢性乙型肝炎病毒(HBV)感染以HBV特异性CD8+ T细胞功能失调为特征,恢复其效应活性是主要的治疗目标。在这里,我们生成了hbv特异性CD4+ T细胞受体转基因小鼠,以证明CD4+效应T细胞可以预防和逆转肝细胞启动诱导的CD8 + T细胞功能障碍。这种拯救增强了抗病毒CD8+ T细胞功能并抑制病毒复制。CD4+ T细胞帮助直接发生在肝脏内,不依赖于次级淋巴器官,需要局部抗原识别。库普弗细胞,而不是树突状细胞,是关键的抗原呈递平台。CD4+ T细胞通过CD40-CD40L相互作用激活Kupffer细胞,触发白细胞介素(IL)-12和IL-27的产生。IL-12扩大CD4+ T细胞库,而IL-27对CD8+ T细胞的救援至关重要。在小鼠和从慢性感染患者分离的T细胞中,外源性IL-27类似地恢复hbv特异性CD8+ T细胞功能。这些发现确定了IL-27是慢性HBV感染的一个可处理的免疫治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
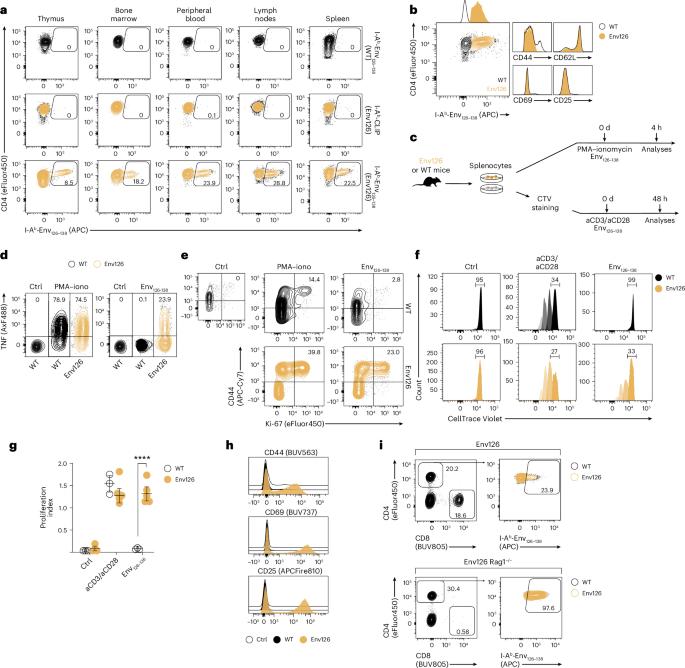
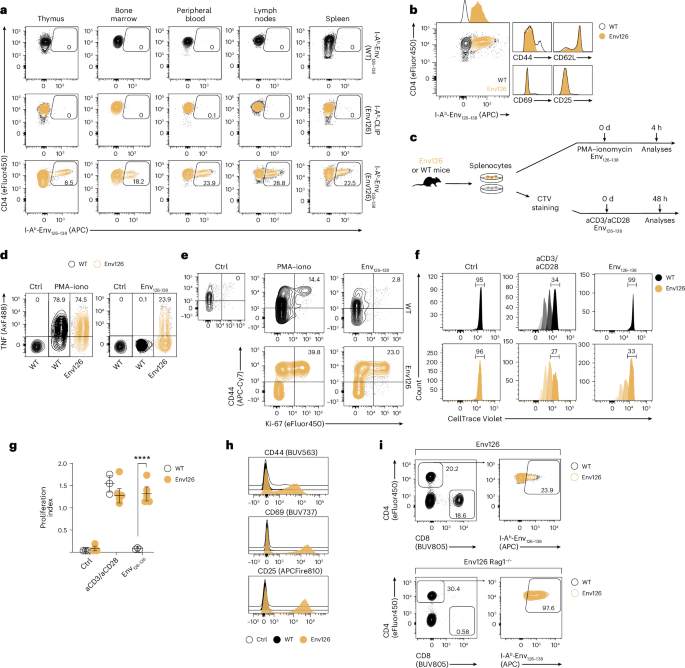
CD4+ T cells license Kupffer cells to reverse CD8+ T cell dysfunction induced by hepatocellular priming
Chronic hepatitis B virus (HBV) infection is marked by dysfunctional HBV-specific CD8+ T cells, and restoring their effector activity is a major therapeutic goal. Here, we generated HBV-specific CD4+ T cell receptor transgenic mice to show that CD4+ effector T cells can prevent and reverse the CD8⁺ T cell dysfunction induced by hepatocellular priming. This rescue enhances antiviral CD8+ T cell function and suppresses viral replication. CD4+ T cell help occurs directly within the liver, independent of secondary lymphoid organs, and requires local antigen recognition. Kupffer cells, rather than dendritic cells, are the critical antigen-presenting platform. CD4+ T cells license Kupffer cells via CD40–CD40L interactions, triggering interleukin (IL)-12 and IL-27 production. IL-12 expands the CD4+ T cell pool, while IL-27 is essential for CD8+ T cell rescue. Exogenous IL-27 similarly restores HBV-specific CD8+ T cell function in mice and in T cells isolated from chronically infected patients. These findings identify IL-27 as a tractable immunotherapeutic target in chronic HBV infection. Here the authors show that CD4+ effector T cells prevent or reverse CD8+ T cell dysfunction by licensing Kupffer cells to trigger IL-27 production, defining a liver-specific immune circuit and a potential target for chronic HBV therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


