人体时钟增强新皮层功能
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
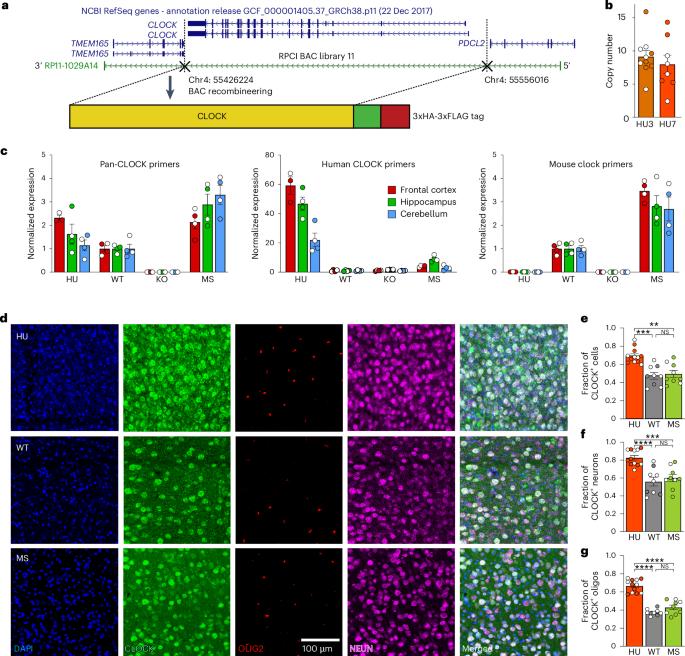
转录因子CLOCK在昼夜节律中无所不在地表达,而其在人类新皮层中的特异性表达表明其具有其他功能。在这里,我们建立了一个小鼠模型(HU),再现了人类皮层中CLOCK的表达。HU小鼠表现出增强的认知灵活性,这可能与CLOCK时空表达的改变有关。细胞类型特异性基因组图谱鉴定了HU小鼠兴奋性神经元中与树突生长和脊柱形成相关的上调基因。我们还发现,HU小鼠的兴奋性神经元树突复杂性和脊柱密度增加,兴奋性突触后电流频率更高,表明神经连通性更丰富。相比之下,在人类诱导的多能干细胞来源的神经元中,CLOCK敲除显示树突复杂性降低,突触前点密度降低。总之,我们的数据表明,CLOCK可能通过改变时空基因表达而进化出与大脑相关的功能增益,这些功能可能是人类大脑特化的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Human CLOCK enhances neocortical function
The transcription factor CLOCK is ubiquitously expressed and important for circadian rhythms, while its human-specific expression in neocortex suggests additional functions. Here, we generated a mouse model (HU) that recapitulates human cortical expression of CLOCK. The HU mice show enhanced cognitive flexibility, which might be associated with alteration in spatiotemporal expression of CLOCK. Cell-type-specific genomic profiling identified upregulated genes related to dendritic growth and spine formation in excitatory neurons of HU mice. We also found that excitatory neurons in HU mice have increased dendritic complexity and spine density, and a greater frequency of excitatory postsynaptic currents, suggesting a greater abundance of neural connectivity. In contrast, CLOCK knockout in human induced pluripotent stem cell-derived neurons showed reduced complexity of dendrites and lower density of presynaptic puncta. Together, our data demonstrate that CLOCK might have evolved brain-relevant gains of function via altered spatiotemporal gene expression and that these functions may underlie human brain specializations. Regulation of gene expression is a facet of human brain specialization. Here, the authors show that human-like expression of the CLOCK gene in the mouse neocortex enhances cognitive flexibility and neural connectivity, suggesting an evolutionary gain of function that may have contributed to human cognitive specialization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


