有合并症患者的COVID-19管理。
摘要
新型冠状病毒病2019 (COVID-19)可导致严重的呼吸系统疾病和相关疾病。弱势群体,包括那些患有慢性阻塞性肺病、心脏病、糖尿病、慢性肾病、肥胖和老年人的人群,面临着严重并发症的风险增加。随着大流行的发展,有各种诊断技术可用于检测严重急性呼吸窘迫综合征(SARS-CoV-2),包括临床表现、快速抗原/抗体检测、分子检测、补充实验室分析和成像。根据同行评议的数据,治疗方案包括恢复期血浆输注、皮质类固醇、抗病毒药物和免疫调节药物。恢复期血浆治疗历来用于中东呼吸综合征、埃博拉和SARS等疫情,世界卫生组织建议在没有疫苗或抗病毒药物的情况下,对COVID-19危重患者采用恢复期血浆治疗。恢复期血浆中的中和抗体有助于控制病毒载量和改善患者预后,特别是在早期给药时,尽管效果各不相同。美国食品和药物管理局已授权将其紧急用于COVID-19重症病例,但输血反应和输血相关急性肺损伤等潜在风险需要进一步调查,以确定确切的疗效。腺苷核苷酸类似物瑞德西韦(Remdesivir)等抗病毒药物抑制病毒RNA聚合酶,并已显示出降低COVID-19严重程度的功效,因此被授权用于住院患者的紧急使用。其他抗病毒药物,如利托那韦、洛匹那韦和乌米非诺韦,会破坏病毒的复制和进入,但它们对SARS-CoV-2的有效性仍在调查中。康复试验显示,地塞米松是一种皮质类固醇,已被用于COVID-19危重患者,以减少炎症和预防呼吸衰竭。其他免疫抑制剂如鲁索利替尼、巴西替尼和秋水仙碱有助于调节免疫反应,减少细胞因子风暴和炎症相关并发症。然而,皮质类固醇具有高血糖、免疫抑制和病毒清除延迟等风险,需要谨慎给药。临床研究的系统回顾显示,羟氯喹联合或不联合阿奇霉素并不能降低病毒载量,也不能减轻症状的严重程度,但会增加急性住院患者的死亡率。与标准治疗相比,15天后患者的临床状况没有改善。美国食品和药物管理局已经撤销了在COVID-19患者中使用羟氯喹的授权,原因是利益-风险平衡为零。正在研究伊托单抗、吉姆西单抗、沙利单抗和托珠单抗等单克隆抗体减轻COVID-19患者严重炎症反应的能力,特别是细胞因子释放综合征和急性呼吸窘迫综合征。这些抗体针对特定的免疫途径来减少促炎细胞因子,其中一些在临床试验中显示出有希望的结果,尽管它们的用途仍在研究中。从许多covid -19阳性患者中测序的簇状规则间隔短回文重复序列/Cas13酶家族可能抑制SARS-CoV-2的复制,切割RNA基因组,并有助于基因组分析的扩增。Cas13也可以通过腺相关病毒载体递送到受感染的肺部,靶向新出现的病原体。除了药物作用外,疫苗还能有效预防有症状的感染,减少住院治疗,降低死亡率,并最终降低疾病的严重程度。本文旨在探讨对患有基础疾病的COVID-19患者的管理,以减轻医疗保健系统的负担。
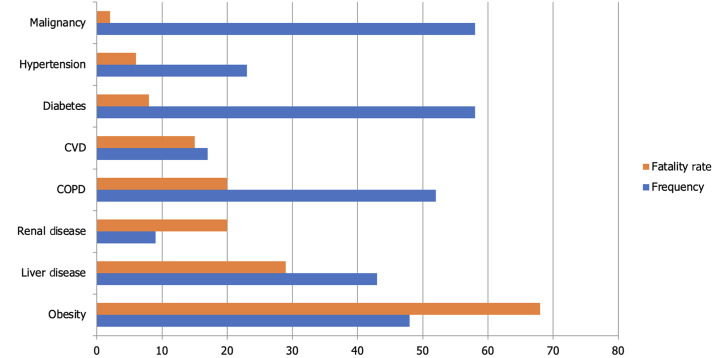
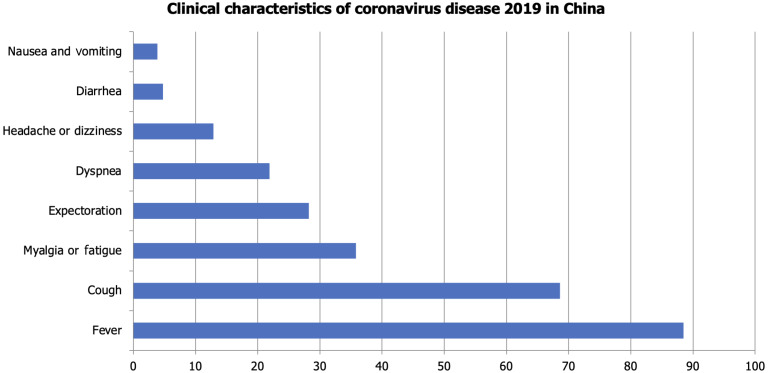
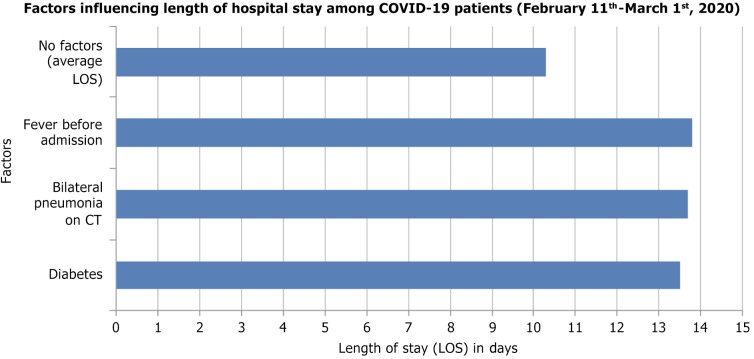
The novel coronavirus disease 2019 (COVID-19) causes serious respiratory illness and related disorders. Vulnerable populations, including those with chronic obstructive pulmonary disease, heart disease, diabetes, chronic kidney disease, obesity, and the elderly, face an increased risk of severe complications. As the pandemic evolves, various diagnostic techniques are available to detect severe acute respiratory distress syndrome (SARS-CoV-2), including clinical presentation, rapid antigen/antibody testing, molecular testing, supplemental laboratory analysis, and imaging. Based on peer-reviewed data, treatment options include convalescent plasma transfusion, corticosteroids, antivirals, and immunomodulatory medications. Convalescent plasma therapy, historically used in outbreaks like Middle East respiratory syndrome, Ebola, and SARS, is suggested by the World Health Organization for critically ill COVID-19 patients when vaccines or antiviral drugs are unavailable. Neutralizing antibodies in convalescent plasma help control viral load and improve patient outcomes, especially when administered early, though effectiveness varies. The United States Food and Drug Administration has authorized its emergency use for severe COVID-19 cases, but potential risks such as transfusion reactions and transfusion-related acute lung injury require further investigation to establish definitive efficacy. Antiviral agents like Remdesivir, an adenosine nucleotide analog, inhibit viral RNA polymerase and have shown efficacy in reducing COVID-19 severity, leading to its emergency use authorization for hospitalized patients. Other antivirals like ritonavir, lopinavir, and umifenovir disrupt viral replication and entry, but their effectiveness against SARS-CoV-2 remains under investigation. Dexamethasone, a corticosteroid, has been used in critically ill COVID-19 patients to reduce inflammation and prevent respiratory failure, as shown in the RECOVERY trial. Other immunosuppressants like ruxolitinib, baricitinib, and colchicine help modulate the immune response, reducing cytokine storms and inflammation-related complications. However, corticosteroids carry risks such as hyperglycemia, immunosuppression, and delayed viral clearance, requiring careful administration. Systematic reviews of clinical studies revealed that hydroxychloroquine with or without azithromycin did not decrease viral load nor reduce the severity of symptoms, but increased mortality among acutely hospitalized patients. There was no improvement in patients' clinical conditions after 15 days compared to standard treatment. The United States Food and Drug Administration has revoked the authorization for the use of hydroxychloroquine in COVID-19 patients due to the null benefit-risk balance. Monoclonal antibodies like itolizumab, gimsilumab, sarilumab, and tocilizumab are being studied for their ability to reduce the severe inflammatory response in COVID-19 patients, particularly cytokine release syndrome and acute respiratory distress syndrome. These antibodies target specific immune pathways to decrease pro-inflammatory cytokines, with some showing promising results in clinical trials, though their use remains under investigation. The Clustered Regularly Interspaced Short Palindromic Repeats/Cas13 family of enzymes, sequenced from many COVID-19-positive patients, can potentially inhibit SARS-CoV-2 replication, cleave the RNA genome, and aid in the amplification of the genome assay. Cas13 can also target emerging pathogens via an adeno-associated virus vector when delivered to the infected lungs. In addition to pharmacological agents, vaccines effectively prevent symptomatic infection, reduce hospitalizations, minimize mortality rates, and ultimately reduce the severity of the disease. This paper aims to explore the management of patients with underlying conditions who present with COVID-19 to lessen the burden on healthcare systems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


