右美托咪定预防心脏手术后谵妄:一项最新的系统评价和荟萃分析与试验序列分析。
IF 4.7
3区 医学
Q1 ANESTHESIOLOGY
引用次数: 0
摘要
背景:术后谵妄仍然是心脏手术后常见的并发症。右美托咪定(DEX)对预防术后谵妄的影响仍然存在争议,因为最近的随机对照试验(rct)给出了相互矛盾的结果。方法:我们对评价DEX预防心脏手术后谵妄疗效的随机对照试验进行了最新的系统回顾和荟萃分析。MEDLINE、Embase和Cochrane数据库的系统检索确定了在年龄≥18岁的患者中比较DEX与安慰剂或其他治疗的随机对照试验。敏感性、亚组分析和试验序贯分析(TSA)评估了研究结果的稳健性。结果:共纳入31项随机对照试验,共纳入5628例患者,其中50.1%的患者使用DEX。DEX组谵妄发生率显著降低(RR 0.61;95% ci, 0.49-0.75;结论:右美托咪定可显著减少心脏手术后谵妄,TSA证实有中度证据。虽然它显示了临床益处,但需要仔细监测心动过缓。系统评价方案:PROSPERO (CRD42024593472)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
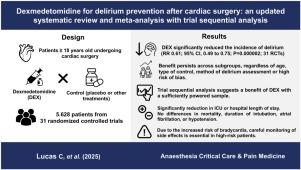
Dexmedetomidine for delirium prevention after cardiac surgery: An updated systematic review and meta-analysis with trial sequential analysis
Background
Postoperative delirium remains a common complication after cardiac surgery. The impact of dexmedetomidine (DEX) on preventing postoperative delirium is still controversial as recent randomized controlled trials (RCTs) have presented conflicting results.
Methods
We conducted an updated systematic review and meta-analysis of RCTs evaluating DEX efficacy in preventing delirium after cardiac surgery. A systematic search of MEDLINE, Embase, and Cochrane databases identified RCTs comparing DEX with placebo or other treatments in patients aged ≥18 years. Sensitivity, subgroup analyses, and trial sequential analysis (TSA) assessed the robustness of the findings.
Results
A total of 31 RCTs including 5628 patients were analyzed, 50.1% of them receiving DEX. Delirium incidence was significantly lower in the DEX group (RR 0.61; 95% CI, 0.49–0.75; P < 0.001). This protective effect remained across subgroup analyses based on age, control type, delirium assessment method, and after excluding trials at high risk of bias. DEX use was associated with a shorter intensive care unit length of stay (MD −0.14 days; 95% CI, −1.28 to −0.04; P < 0.01). TSA confirmed the result’s robustness. However, DEX increased bradycardia risk (RR 1.53; 95% CI, 1.05–2.21; P = 0.02). No significant differences were found in mortality, intubation duration, hospital length of stay, atrial fibrillation, or hypotension.
Conclusions
Dexmedetomidine significantly reduces postoperative delirium following cardiac surgery, with moderate evidence confirmed by TSA. While it demonstrates clinical benefits, careful bradycardia monitoring is warranted.
Systematic review protocol
PROSPERO (CRD42024593472).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Anaesthesia Critical Care & Pain Medicine
ANESTHESIOLOGY-
CiteScore
6.70
自引率
5.50%
发文量
150
审稿时长
18 days
期刊介绍:
Anaesthesia, Critical Care & Pain Medicine (formerly Annales Françaises d''Anesthésie et de Réanimation) publishes in English the highest quality original material, both scientific and clinical, on all aspects of anaesthesia, critical care & pain medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


