光/铜(I)协同催化苯乙烯的三组分自由基单氟烷基芳基化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在光/铜(I)协同作用下,建立了苯乙烯与溴氟乙酸乙酯和(杂)芳烃的三组分自由基单氟烷基芳化反应。在一步反应中,可以构建两个新的碳-碳键和1,1-二(杂)芳基骨架。该反应具有反应条件温和、产率好、底物范围广、区域选择性好等特点。此外,该方法适用于复杂分子的构建和大规模合成,为天然产物和药物的后期单氟化修饰提供了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
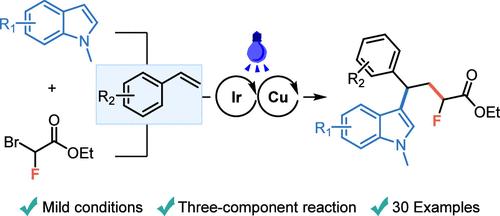
Light/Copper(I) Synergistically Catalyzes the Three-Component Radical Monofluoroalkyl Arylation of Styrenes
A three-component radical monofluoroalkyl arylation reaction of styrenes with ethyl bromofluoroacetate and (hetero)arenes catalyzed by light/copper(I) synergy is established herein. In a one-step reaction, two new C–C bonds and 1,1-di(hetero)aryl skeletons can be constructed. This reaction has the characteristics of mild reaction conditions, good yields, a wide range of substrates, and good regioselectivity. In addition, this method is suitable for the construction and large-scale synthesis of complex molecules, providing a new avenue for the late monofluorination modification of natural products and drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


