通过α-氨基酰化/消除异氰酸酯序列的吲哚酮衍生物的β-芳基化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
β-芳基羰基化合物是药物和生物活性分子的重要基序,但从饱和羰基化合物中合成它们仍然是一个重大挑战。我们报告了一种实用且操作简单的环羰基化合物β-芳基化方案,该方案与α-功能化方法无缝集成,能够快速、立体选择性地构建多取代羰基化合物。这种β-芳基化方案的简单性、广泛的适用性和机理见解使其成为复杂羰基框架的流线型合成的有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
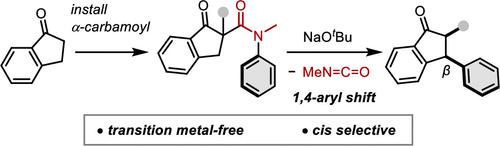
β-Arylation of Indanone Derivatives via α-Carbamoylation/Elimination of Isocyanate Sequence
β-Aryl carbonyl compounds are crucial motifs in pharmaceuticals and bioactive molecules, yet their synthesis from saturated carbonyl compounds remains a significant challenge. We report a practical and operationally simple protocol for β-arylation of cyclic carbonyl compounds, which seamlessly integrates with α-functionalization methods, enabling rapid, stereoselective construction of multisubstituted carbonyl compounds. The simplicity, broad applicability, and mechanistic insights provided by this β-arylation protocol establish it as a valuable tool for streamlined synthesis of complex carbonyl frameworks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


